The Orbital Approach to the irreversible addition of organometallics to the Carbonyl Group - Taking Things a Little Further
The description of the irreversible nucleophilic addition of either organometallic reagents or reduction with hydride-based reducing reagents on the main blog page should be enough for most introductory organic courses. Those that take organic chemistry further might be interested in the orbitals involved. I have effectively shown this already. The general orbital description is the same for all these addition reactions. The only minor difference is how we draw the HOMO (highest occupied molecular orbital) of the nucleophile.
The organolithium is the nucleophile with the C–Li bond polarized towards the carbon as indicated by the larger size of the orbital close to the carbon. This interacts with the empty π* C=O antibonding orbital. This bond is also polarized so that the larger orbital coefficient is on carbon.
The organolithium reagent is the nucleophile. It donates two electrons from a bonding orbital. This is shown by overlapping the C–Li σ bond with the empty π* antibonding orbital of the the carbonyl group.
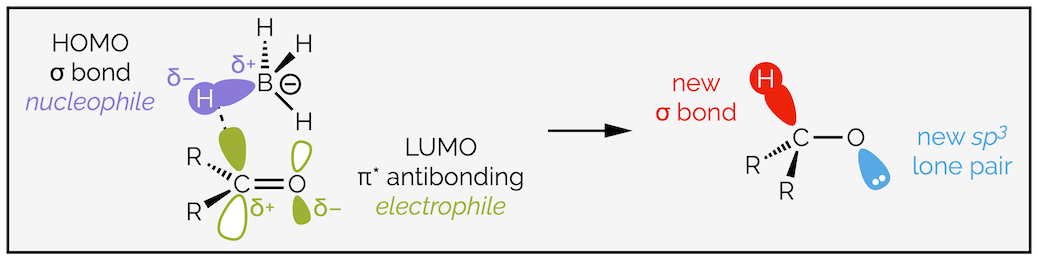
The overlap of the σ bond of the borohydride anion with the π* antibonding orbital of the electrophilic carbonyl group. This leads to the formation of a new σ bond and a new lone pair of electrons on the electronegative oxygen atom.
The hydride is the same, there is an overlap between the HOMO of the nuclelphile and the LUMO of the electrophile. The HOMO is the polarized B–H σ bond and the LUMO is the carbonyl π* antibonding orbital. When the two orbitals overlap a new σ bond is formed between the C–H while filling the antibonding orbital leads to the C=O double bond breaking.