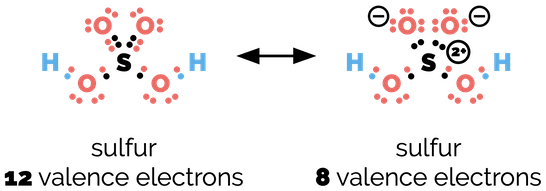Lewis Structures
Introduction
Organic chemistry is about making molecules with new properties. To make molecules we must make bonds, and to do this we must know where the electrons are in a molecule.
Organic chemists use many different models to represent molecules and the distribution of electrons. The more accurate the model, the more complex it tends to be. It turns out that an incredibly simplistic model is sufficient to enable chemists to plan syntheses, and rationally design new molecules.
The simplest representations are all based on Lewis structures. These show the outer or valence electrons of each atom, and allow chemists to predict both the structure and reactivity of molecules. The Lewis structure is basically the code for a molecule. From this code, we can build all our other concepts and models.
Your rarely see Lewis structures in books. They are messy and time-consuming to draw, but they are the foundation of all our other representations. At the start of your chemical eduction, they are invaluable, they are like training-wheels, helping you understand complex principles. But, like training-wheels, you will rapidly out grow them.
Atoms
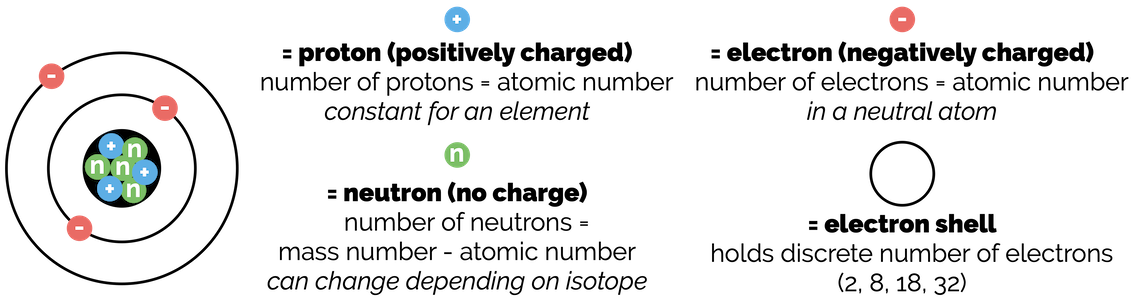
When most of us think about the structure of an atom, we visualise what is known as the Bohr model of an atom. In the Bohr model there is a central nucleus that comprises of positively charged protons and uncharged neutrons. Surrounding the nucleus are electron shells, planetary-like orbitals that contain a discrete number of electrons.
Simplified representation of an atom (the Bohr model)
The periodic table tells you about the composition of each atom. The number of protons in the nucleus is given by the atomic number. The number of neutrons is found by subtracting the atomic number from the mass number. In a neutral, non-charged atom, the number of electrons is also equal to the atomic number.
Valence electrons
Each electron shell surrounding the nucleus can hold a specific number of electrons. This is a result of electrons being both particles and waves, but we don’t need to think about this at the moment. Electrons fill the shells from the bottom up. That is they start with less energy or nearer the nucleus. The first shell can contain 2 electrons. The second shell can contain 8 electrons. The third can contain 18.
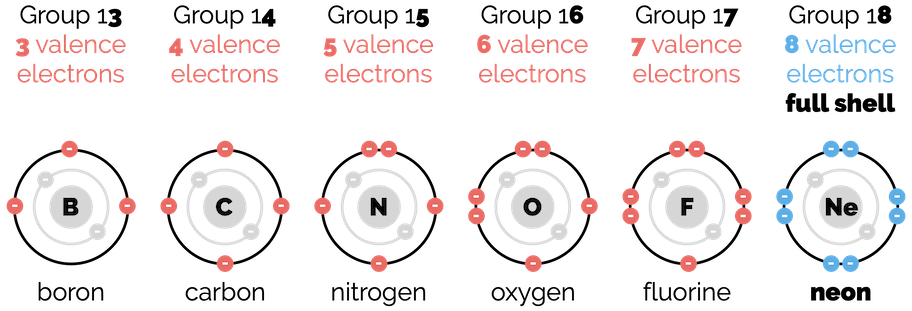
But for Lewis structures, we are only concerned with the electrons in the outer shell. These are the electrons that will form bonds. They are known as valence electrons. Once again, the periodic table tells us how many valence electrons each neutral atom has. It is given by the group number as shown in the diagram below:
Number of valence electrons along the second row of the periodic table
With the exception of the noble gases (group 18), all the atoms are reactive and will form bonds with other atoms. What makes the noble gases so special? They have a full outer shell. If they lose electrons the shell is incomplete while if they gained electrons they would have to start a new shell. Neither of these outcomes is particularly attractive and they are stable as single atoms.
All the other atoms react to gain an electron configuration the same as a noble gas. In other words, all the other atoms react so that they have a full outer shell. For the majority of organic chemistry, this means atoms should have eight valence electrons, the same as neon.
Bonds
There are two kinds of bond, ionic bonds and covalent bonds. In an ionic bond one atom loses electrons and the another gains electrons. This results in one species becoming positively charged (it has more protons than electrons) and the other becoming negatively charged (it has more electrons than protons). There is an electrostatic attraction between the opposite charges. This is an ionic bond.
An example of an ionic bond
The most frequently encounter ionic bonds are formed when a metal loses electrons, emptying its outer shell or falling back to the electron configuration of the previous noble gas. The resulting positively charged species or ion is known as a cation. The other species gains electrons, filling its outer shell and jumping forward to the electron configuration of the next noble gas. This negatively charged ion is called an anion.
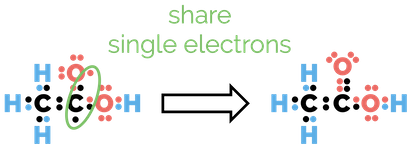
Covalent bonds are formed when two atoms share electrons. Each covalent bond comprises of two electrons. An atom will form as many covalent bonds as it needs to gain a full outer shell or the electron configuration of a noble gas.
Examples of covalent bonds as found in hydrogen and methane
In organic chemistry, understanding covalent bonds is essential. Lewis structures help us to see how many bonds an atom will form. Before we look at drawing Lewis structures we have to answer one question. How do we know which bond is going to form, ionic or covalent?
Each atom has a different affinity or attraction to electrons. The more an atom wants an electron, the more electronegative it is said to be. Once again, the periodic table is very useful when considering electronegativities. Electronegativity increases along a row of the periodic table and it increases up a group of the periodic table. This means atoms on the top right corner (ignoring the noble gases) are the most electronegative and those in the bottom left are the least.
Whether an ionic or covalent bond is formed can be judged by looking at the difference in electronegativity between two atoms. If there is a large difference in electronegativity (the atoms are form different sides of the periodic table), then one atom wants an electron a lot more than the other and an ionic bond is formed. If the electronegativities are similar, neither atom wants the electron enough to take it, then the electrons are shared and a covalent bond is formed.
Lewis structures
There are a number of strategies for drawing Lewis structure depending on whether you are looking at organic molecules or inorganic molecules. As we will be dealing with organic compounds we will look at a simple approach that works well for these molecules.
There are two helpful guidelines to drawing Lewis structures of organic molecules.
Electrons come in pairs (bonds or lone pairs of electrons)
You are aiming to obey the octet rule or have eight valence electrons
Let’s draw the Lewis structure of acetic acid (ethanoic acid; C2H4O2)
Step 1 - Atoms: draw the atoms
First we need to know how many atoms we have and how many valence electrons each one has
Step 2 - Skeleton: join atoms that can form more than one bond
Next we join the atoms that can form more than one bond. It is most common to start with the carbon atoms and then decorate these with other atoms. There may be more than one way to combine the atoms (see section on isomers below). Don’t worry about joining the wrong atoms - as long as you don’t break the rules we are listing, you will have a valid structure.
Step 3 - Hydrogens: add hydrogen atoms
Add any halogen or hydrogen atoms. It is best to start by placing them on the atom with the most unpaired electrons first. The goal is to get each atom to a full outer shell. This is invariably the fabled eight electrons of the octet rule.
Step 4 - Multiple bonds: the octet rule
Next check that every atom obeys the octet rule (except hydrogen which will have two electrons). If an atom has less than eight electrons then try and share electrons with an adjacent atom to create a multiple bond. In this example, one oxygen and one carbon have 7 valence electrons. Forming a double bond solves this problem.
Step 5 - Simplification
Lewis structures are messy. Each pair of shared electrons, a covalent bond, can be replaced by a line connecting the atoms. But keep the lone pair of electrons visible for the time being. These are important for shape and reactivity.
Structural Isomers and Resonance Structures
When drawing a Lewis structure, you may not be told the structure or connectivity. In these cases, there may be more than one allowable Lewis structure, a structure in which all the atoms obey the octet rule. This is can arise for two reasons: isomers and resonance structures.
Structural isomers are different arrangements of the same atoms. The isomers will have the same molecular formula but different connectivity or constitution. Below is an isomer of acetic acid, the compound we drew above.
Structural isomers of acetic acid
Resonance structures are slightly more confusing. Again our atoms obey the octet rule and the structure is largely the identical. The structures differ by the position of multiple bonds or lone pairs of electrons. This arises as the electrons of certain molecules are said to be delocalised. They are are found in multiple positions. We will cover this in more detail in a later summary.
An example of delocalisation - two resonance structures of acetic acid
Warning - the octet rule
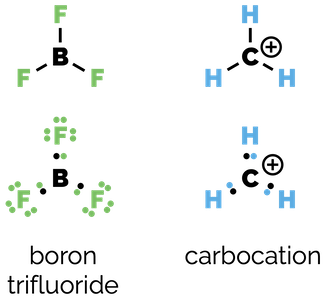
Organic chemists seem to be obsessed by the octet rule but there are numerous examples where it does not hold true. The octet rule is only true for the second row of the periodic table where the electron configuration of neon represents a stable state. Elements of the second row will never have more than eight valence electrons but they can have less. For example neutral compounds of boron or positively charged carbon species.
Less then the octet rule - species with six valence electrons
Elements of the third row of the period table frequently obey the octet rule and have the electron configuration of argon. But the third shell of an atom can contain more electrons. It is possible to have 18 electrons in this shell. In some molecules, a third row element will have more than eight electrons such as the sulfur in sulfuric acid.
Breaking the octet rule - one resonance structure of sulfuric acid exceeds 8 valence electrons
Conclusion - what good are Lewis structures?
Lewis structures are basically ‘training wheels’ that help you determine the position of valence electrons around the atoms of a molecule. Over time, you will find that you need them less and that simplified representations of molecules give sufficient information for you to know the placement of the electrons. We need to know about valence electrons for three reasons: 1. they tell us how many bonds will be formed; 2. they help us determine the geometry of the atoms; 3. they show the location of lone pairs of electrons.
When we turn our attention to reactions, you shall see that each of these three factors are very important in order to determine reactivity.