Thin Layer Chromatography (TLC)
Introduction
Thin layer chromatography (TLC) is a chromatographic technique that separates the components of a mixture. It is incredibly useful as it is simple, fast, very sensitive, and relatively cheap. In many respects, TLC is the archetypal chromatographic technique. The basic principles covered here act as an introduction to more advanced techniques, such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), that are used in many industries for quality control purposes.
While preparative TLC does allow the small scale separation and isolation of compounds, TLC is normally used as an analytical technique either to assess the purity of a sample or follow a chemical reaction.
The Practical Side of TLC
There are three elements to all chromatographic systems. There is the sample (or analyte), the mixture of compounds to be analysed. There is a stationary phase, the material that separates the components of the mixture, and finally, there is the mobile phase, which moves through the stationary phase and carries the sample across it.
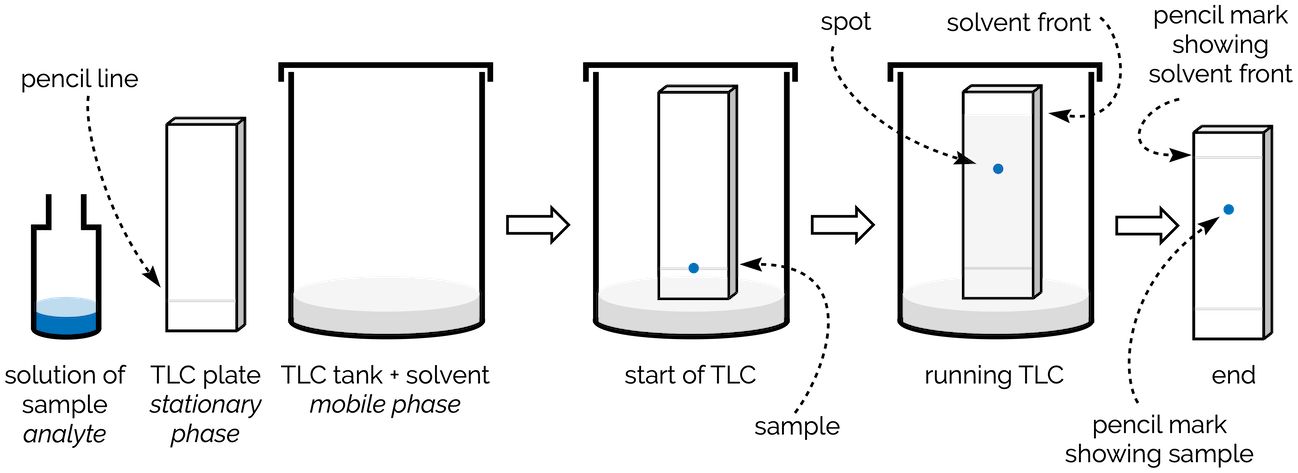
In TLC, the stationary phase is an aluminium or glass plate covered in silica (often impregnated with a florescent dye to aid visualisation). This is cut into a rectangle and a pencil line is drawn about a centimetre from the bottom. This is the baseline. The sample is dissolved in a volatile solvent and then applied as small dot on the baseline.
Cartoon of running a TLC plate
The mobile phase is a solvent or mixture of solvents that will carry the samples up the TLC plate. It is placed in a TLC tank (beaker or jam jar) so that it just covers the bottom (it must be less than 1 cm deep). The TLC plate is then carefully lowered into the tank so that the level of the solvent/mobile phase is below the baseline. The tank is covered.
The solvent slowly moves up the TLC plate (it is pulled up by capillary action). As it travels up the plate, the various components of the samples travel at different speeds and begin to separate. This is known as developing the TLC plate. Once the solvent gets close to the top of the plate, the plate is removed from the tank and left to dry. There are various techniques to visualise the spots on the TLC. If the compounds are coloured you can simply see them. Most organic compounds are not coloured and so other techniques to see them are required. The most common is to view the plate under a UV lamp. In most commercial TLC plates the stationary phase is mixed with a dye that appears green under UV light. If the components of the sample contain conjugation they will appear as a dark shadow that doesn't glow under UV light. There are many other chemical methods to visual the compounds by making them react on the plate (iodine and potassium permanganate solution are two of the most common).
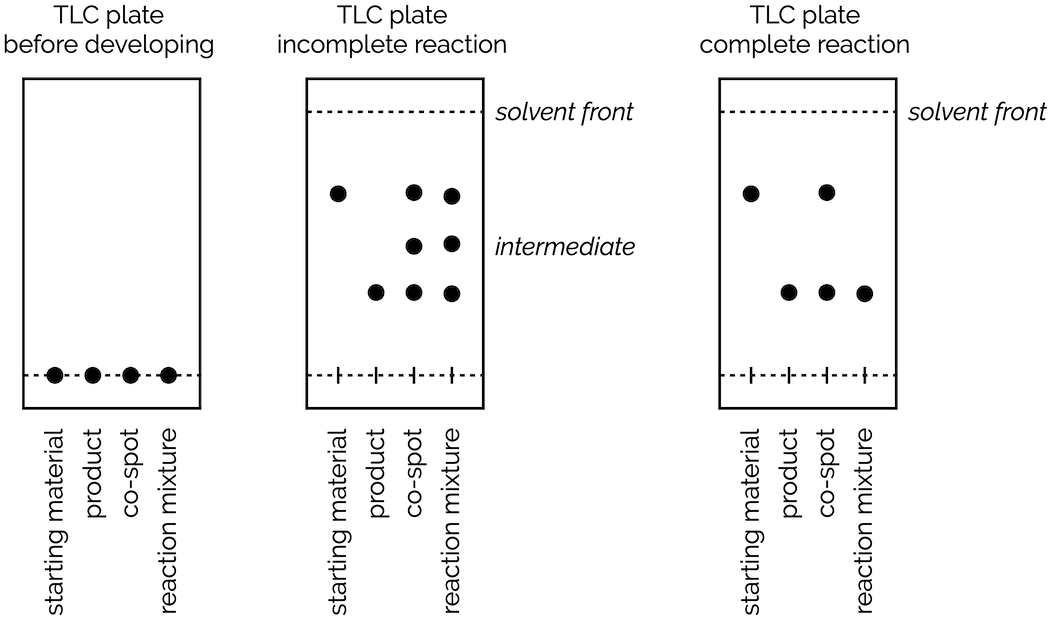
A typical TLC plate for following a reaction is shown below. In this example, there are four different spots on the baseline; one for the starting material, one for the product, a co-spot that is made up of the other three spots and finally a spot for the reaction mixture. The co-spot aids identification of the different samples and ensures that they run in the same environment (so takes care of experimental error).
Following a reaction by TLC. From left to right: Before running the TLC all spots are on the baseline. Second diagram shows the TLC plate for a reaction that still has starting material and an intermediate present. The reaction has not gone to completion. The final diagram shows a reaction in which all starting material has been consumed and converted to product.
The distance the spots have moved from the baseline is normally recorded as a retention factor (Rf value). This is given by:
Determining the R<sub>f</sub> value of a sample
Once a TLC plate has been run, a pure compound will show as a single spot. Most mixtures will appear as multiple spots, with a spot for each component of the mixture. Occasionally, two compounds will have the same Rf value in a particular solvent (mobile phase). But the opposite is never true (at undergraduate level), multiple spots will never indicate a pure compound.
Examples of TLC plates - frequently a single spot is a pure compound but occasionally you can be unlucky and two different compounds have the same Rf value.
Basic Explanation
This is a simplified explanation as an introduction to chromatography for organic chemistry students. The key to chromatography is the non-covalent interactions between the molecules of the sample and both the stationary and mobile phases. Different molecules will have different interactions and this will cause them to move at different speeds. Strong interactions between the molecules and the stationary phase slow the molecule down while strong interactions with the mobile phase will cause the molecule to move faster.
Interactions between silica stationary phase and sample cause separation
The following factors are generalisations. As with much of chemistry and science, the answers aren’t black & white, and you should balance the influence of each factor.
In TLC the stationary phase is normally silica. This has hydroxyl groups (OH) on the surface and we can make the following generalisations:
Compounds that can hydrogen bond to the hydroxyl groups as either H-bond donors or acceptors will move slower as it is harder to drag them across the stationary phase.
Polar compounds interact strongly with the polar stationary phase and move slower.
Ionic compounds frequently stick to the baseline unless highly polar solvents (mobile phase) are used.
Large molecules are hard to move. They create greater non-covalent interactions.
As for the mobile phase:
The first step in moving a compound is dissolving it. So the more soluble a compound the faster it ends to move.
Insoluble compounds either move very slowly or stick to the baseline.
More polar solvents pull compounds upwards faster.
Conclusion
You will meet TLC in many undergraduate laboratories as either a means of assessing purity or following the progress of a reaction. This summary is simply meant as a brief introduction so that you understand why compounds can be separated. A golden rule of laboratories is ‘if you don’t know what you are doing don’t do it.’ More detailed descriptions can be found on the intertubes.