Aromatic Molecules
Introduction
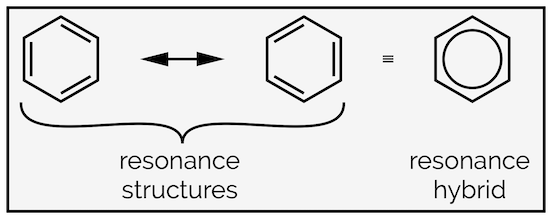
Most high school chemistry students have met benzene and have learnt to draw this molecule as a six-membered ring with a circle in the centre. The reason for this circle is to show that all the bonds are the same length, somewhere in between a single and a double bond. This is actually an example of electron delocalization, and the circle represents the resonance hybrid (see some of the previous summaries). The drawings of benzene with the electrons localized, a series of alternating double bonds, are the resonance structures.
Resonance structures of benzene and the resonance hybrid
Benzene is the archetypal aromatic compound. These are a special class of molecule that contain a ring of delocalized electrons. These molecules are normally more stable than we might predict. This is the result of resonance stabilization or aromatic stabilization. Aromatic compounds are very important and have their own reactivity. In this summary, we will give a simple introduction to aromatic compounds and present some simple rules for predicting which compounds are aromatic.
Aromatic compounds
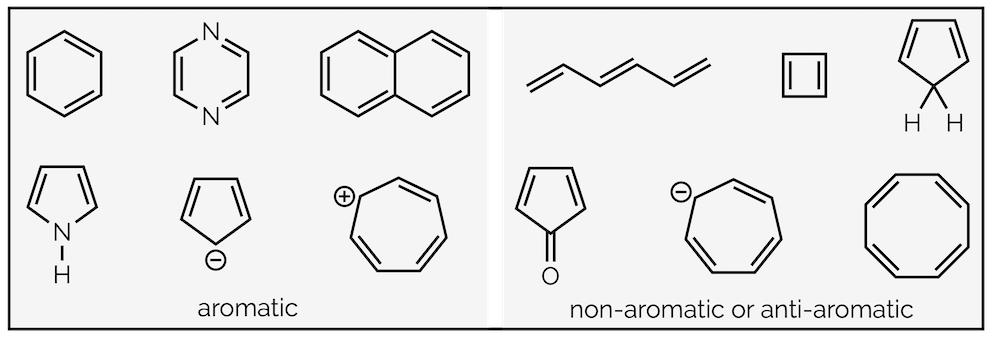
So what makes a molecule aromatic? Below are examples of both aromatic and non-aromatic compounds. What is the common connection between all aromatic compounds?
Examples of aromatic, non-aromatic and anti-aromatic compounds
There are a number of common traits and it turns out that aromatic compounds must satisfy a number of criteria:
They must be cyclic
They must have a ring of continuously overlapping p orbitals (an unbroken ring of conjugated π electrons)
They must have a certain number of π-electrons (known as the Hückel number or obeying Hückel’s rule)
They should be flat
Criteria 1 & 2 - An unbroken ring of conjugated π (pi)-electrons
The first criterion is easy, is the molecule a ring? Benzene is a six-membered ring and is aromatic, hexa-1,3,5-triene is not a ring and is not aromatic.
Benzene is cyclic so can be aromatic, hexatriene is acyclic and cannot be aromatic
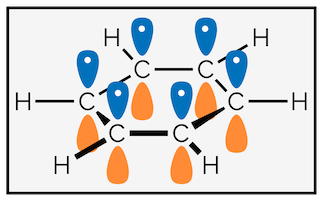
The second factor is harder to see. Benzene has three alternating double bonds (in one of its resonance structures). This is an unbroken circle of conjugation. Multiple resonance structures can be drawn and the curly arrows can be pushed in either direction (it is a ring). There are six 2p orbitals that can overlap in an unbroken circle.
The non-hybridized 2p orbitals of benzene overlap around the ring and show an unbroken ring of conjugation
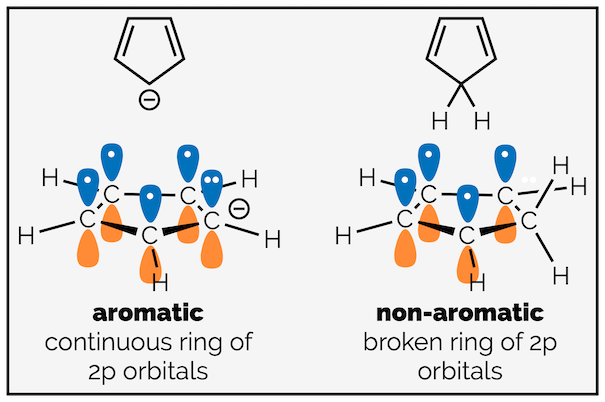
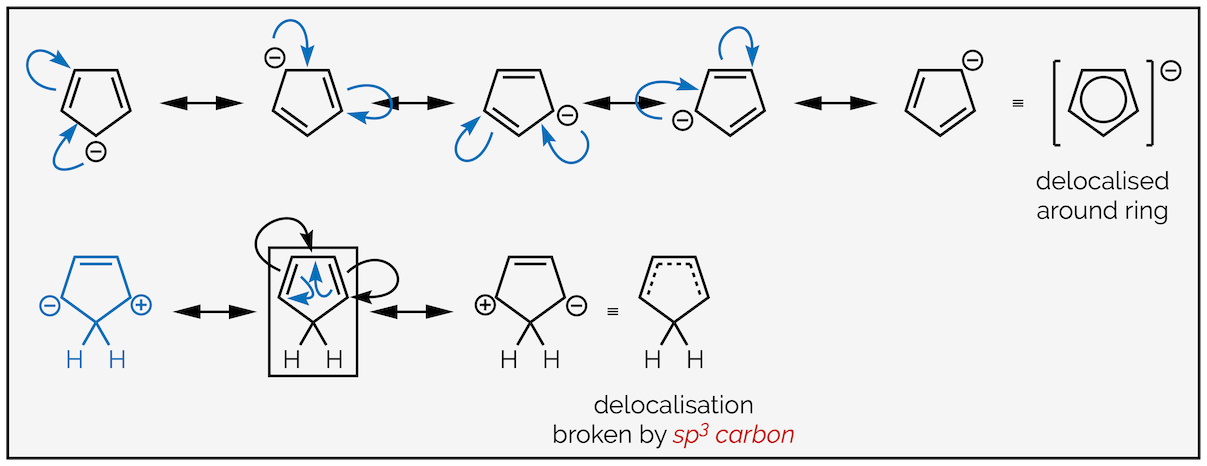
This is easy to see in other six-membered rings like the pyrazine above (or pyridine below). It is often harder to see in other ring sizes. Let’s look at two five-membered rings (below), why is the cyclopentadienyl anion on the left aromatic but cyclopentadiene on the right isn’t? Look at the hybridization of the carbon atoms.
Cyclopentadienyl anion is aromatic with an unbroken ring of 2p orbitals. Cyclopentadiene is not aromatic due to the sp3 carbon breaking the ring of 2p orbitals.
The cyclopentadienyl anion has a lone pair of electrons next to a double bond. These are delocalized (see the summary on valence bond theory). This means there is an unbroken ring of conjugated π-electrons. This can be seen by drawing the resonance structures or looking at the ring of 2p orbitals. Cyclopentadiene has an sp3 hybridized carbon breaking the ring. The electrons cannot be delocalized around the ring.
The delocalization of the cyclopentadienyl anion (top) and the resonance structures for cyclopentadiene highlighting that the π electrons are never delocalized on the carbon
Heterocyclic rings, such as pyrrole, are aromatic for the same reason. Here the lone pair of electrons on the nitrogen atom is adjacent to two double bonds so it is delocalized. This means the nitrogen is sp2 hybridized and not sp3. This allows five 2p orbitals to be in an unbroken circle.
Pyrrole has an unbroken circle of delocalized π electrons
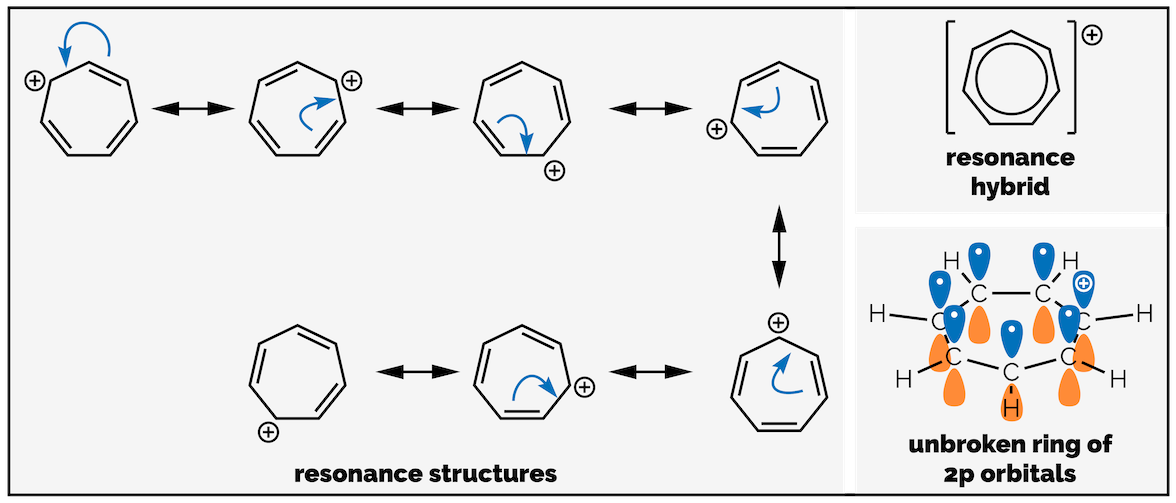
There does not have to be electrons in the 2p orbitals for the compound to be aromatic. The 2p orbitals must be adjacent and ensure that π electrons delocalized in an unbroken circle over the whole ring. An empty p orbital permits delocalization. This should be obvious by inspection of the possible resonance structures in pyrrole (above) and is also shown by the carbocation of cycloheptatriene, another aromatic compound (below).
The cycloheptatrienyl cation is aromatic, with an unbroken circle of π electrons
Criterion 3 - the correct number of π electrons
This is probably the most confusing of the criteria. If a compound obeys the first two criteria and has the correct number of electrons it is aromatic. If it obeys the first two criteria but has the wrong number of electrons it is anti-aromatic. This can be seen if you compare the anion and cation of cyclopentadiene:
The anion of cyclopentadienyl is aromatic, the cation is anti-aromatic
Both compounds are cyclic. Both compounds have unbroken conjugation around the ring with five 2p orbitals on adjacent atoms capable of overlapping, but only the anion on the left is aromatic. This is because it has the correct number of π electrons. What are the correct numbers? Simplistically, aromatic compounds will have an odd number of pairs of π electrons. This means aromatic compounds have 2e- (1 x 22e1), 6e- (3 x 2e-), 10e- (5 x 2e-), 14e- (7 x 2e-) ... π electrons. The series of allowable numbers can be summarized as 2n+2 π electrons, where n = a whole number (but n is not a property of the molecule). This is often known as Hückel’s rule.
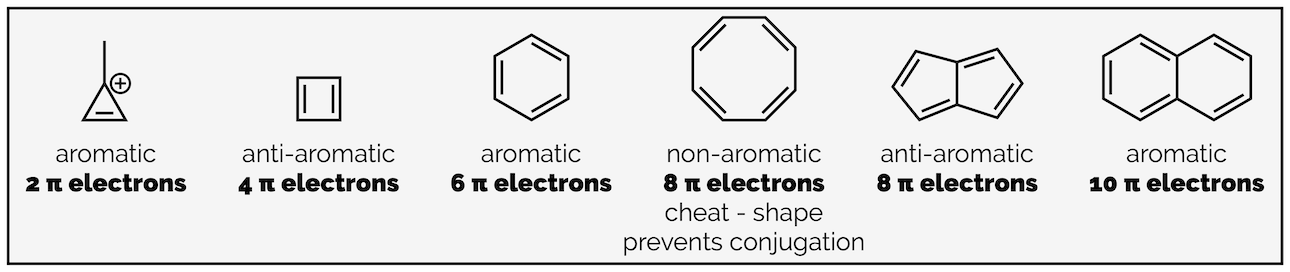
In the diagram below, three compounds obey Hückel’s rule, and are aromatic. Two compounds obey all the criteria for aromaticity bar Hückel’s rule so are anti-aromatic. FInally, one compound is a little odd, as it looks anti-aromatic but is better described as non-aromatic. It doesn’t obey the criteria above and will be used to introduce the fourth criterion:
Aromatic and anti-aromatic compounds (and one non-aromatic compound)
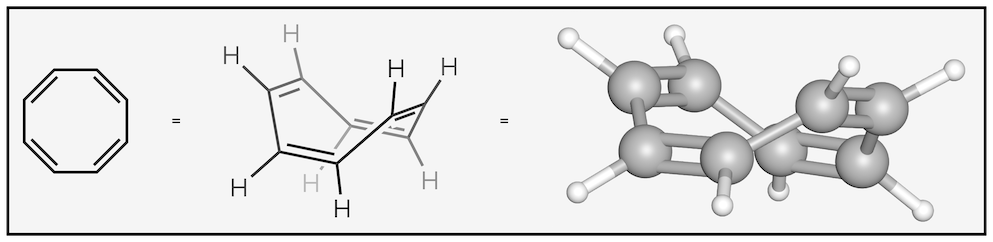
The cation of cyclopropene (first molecule) is aromatic as it has a single pair of π electrons thus obeying Hückel’s rule (2n+2 where n = 0). The next molecule, cyclobutadiene, is anti-aromatic as it has two pairs, or four, π electrons, which is not one of the numbers listed by Hückel’s rule. Benzene is, of course, aromatic. Then comes our little cheat, cyclooctatetraene, this looks like it should be anti-aromatic but is actually best described as non-aromatic. It fails one of the earlier criteria, in that it isn’t an unbroken ring of π electrons. The diagram above makes it look like it is conjugated but the actual shape of the molecule is below. In other words, it isn’t flat and delocalization cannot occur:
Shape of cyclooctatetraene
This shows how important the shape of molecules can be!
After cyclooctatetraene is the first bicyclic compound, this is pentalene and it is anti-aromatic. It is flat (planar) and does have an unbroken ring of π electrons and it has eight π electrons. So it does not obey Hückel’s rule. Finally, there is naphthalene, a planar compound that has an unbroken ring of ten π electrons, one of Hückel’s numbers and an aromatic compound.
Heteroaromatic Rings
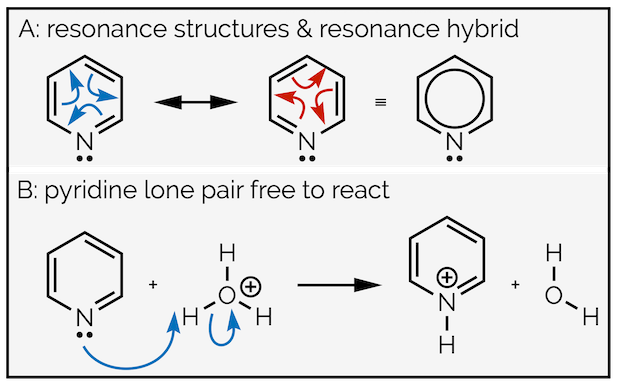
Heteroaromatic compounds replace one or more carbon atoms with a heteroatom. They can cause confusion to the unwary as some have a lone pair of electrons that can react while others lose their lone pair to the aromatic ring. Let’s compare pyridine and pyrrole. Pyridine is an aromatic compound. It has six π electrons. These are clearly identifiable from the double bonds in the resonance structures below. This means the lone pair of electrons on the nitrogen atom is not involved in the aromaticity. It is not delocalized and it is free to react. Pyridine is a good base (proton acceptor).
The lone pair of the nitrogen of pyridine is not involved in the aromatic ring and can react
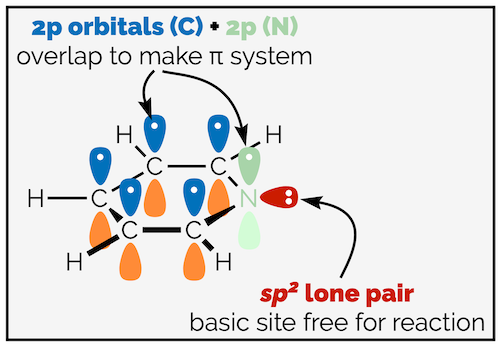
If you were to construct a valence bond representation of pyridine (and if you can’t, read the summary on valence bond theory), you would see that the nitrogen is sp2 hybridised. The lone pair of electrons is in an sp2 hybrid orbital which is at 90° to the 2p orbitals that give the unbroken ring of π electrons that is the aromatic ring. The lone pair cannot overlap with the π electrons.
Valence bond model showing the lone pair of pyridine
Pyrrole is different. There are four π electrons in the double bonds. If this was all the π electrons pyrrole had it would be non-aromatic as the nitrogen atom would break the conjugation, but, as we discussed above, the nitrogen has a lone pair of electrons that can contribute to the π system. This means there are six π electrons and pyrrole is aromatic. It also means that the nitrogen atom’s lone pair is delocalized. It is not free to react and pyrrole is a poor base. In the valence bond representation, the nitrogen is sp2 hybridized again but this time the lone pair is in the 2p non-hybridized orbital.
The lone pair on nitrogen of pyrrole is delocalized and a part of the aromatic ring. It is not basic.
Conclusion
Aromatic compounds are a special class of compound. They are far more stable than we might otherwise predict as a result of delocalization of π electrons or resonance stability. This stability changes their reactivity, they do not behave like alkenes even though we draw double bonds in their resonance structures. Their reactivity is unique and, we often consider aromatic compounds to be their own functional group.
Practice identifying aromatic compounds with this WORKSHEET.