Polarity & Non-Covalent Interactions Part 1
Introduction
Covalent bonds form molecules. Non-covalent interactions between molecules, often known as intermolecular forces (although the same interactions can occur within large molecules), influence the physical properties and the reactivity of molecules. This summary provides a brief overview of electron distribution within a molecule and how this leads to different interactions between molecules. In a second post we’ll look at how these interactions contribute to some physical properties (at a first year level).
But, before we start, a quick disclaimer: The terminology around non-covalent interactions is muddled. Certain phrases mean different things to different chemists (some colleagues would say that a hydrogen bond is a covalent bond …). Not only can this be confusing but occasionally leads to internal inconsistencies. I will undoubtedly add to this. The main problem is van der Waals forces. I’m going to use the IUPAC definition HERE, which is a catch all covering the interactions of uncharged molecules (though, I’ll probably use it incorrectly), but please be aware that many organic chemists use ‘van der Waals forces’ to mean dispersion forces.
Bond Dipoles
The electrons of a covalent bond are rarely shared evenly, being closer to the more electronegative element. This end of bond will be more electron rich, or have a higher electron density, and will be partially negatively charged. This is often indicated by the symbol δ- (see below). The other end will be slightly positively charged, δ+, and is electron poor or has low electron density. This can also be shown by a dipole arrow, where the cross indicates the partial positive charge and the head of the arrow shows the area of high electron density. Bonds with an uneven distribution of electrons are said to be polar.
Polar bonds and the two standard conventions for depicting polarization
Electronegativity is a measure of an atoms ability to attract electrons. It is possible to find extensive tables of values (using the Pauli scale) but there is little point in memorizing these. All you need to remember are the general trends:
electronegativity increases along a row of the periodic table
electronegativity increases on going up a group.
For organic chemistry, all you really need to know is that oxygen, nitrogen, and the halogens (elements to the right of carbon) are more electronegative than carbon. Those to the left, metals, are less electronegative. Also, it is worth noting that alkyl groups are electron releasing, acting as if they were less electronegative.
The polarization of bonds through single or σ bonds is often called the inductive effect. A polar bond is said to have a bond dipole (or even a bond dipole moment. Dipole refers to the separation of charge while ‘moment’ means the distance separating the charges is also important but you don’t really need to know this yet). It can occur over more than one bond although its influence rapidly diminishes the further from the electronegative atom you get.
The inductive effect exerts an influence over several bonds although its strength rapidly drops off (has less effect)
Resonance, or the delocalization, of π electrons also leads to polarization, and this is often known as the mesomeric effect. A typical example of this is found in phenol:
The mesomeric effect - delocalization of electrons and the resulting polarization of the resonance hybrid
Drawing the various resonance structures of a molecule reveals how the electrons are distributed. The formal negative charges indicate where there is a build up of electron density. In the real molecule, the resonance hybrid, these can translate into partial charges that show where bond dipoles exist. These areas effect both the physical properties and the reactivity of phenol. The mesomeric effect is generally stronger than the inductive effect. It is easily communicated over a larger area or a greater distance. It can cover any of the atoms of a conjugated π system.
Molecular dipole
In a polar molecule, one end will be partially negatively charged while the other end is slightly positively charged. This is easy to picture with diatomic molecules such as H–Cl, where the so-called molecular dipole matches the polarity of the bond (the bond dipole). A pole is charged region, so for a molecule to be polar it must have one region of partial positive charge, and one region of partial negative charge or two regions of charge, a dipole.
Diatomic molecule showing the polar bond (bond dipole) and the molecular dipole
For larger molecules, it is necessary to take into account the shape of the molecule. Each bond dipole is added together and, if they do not cancel each other out, then the molecule is polar or has a molecular dipole. For example, the two polar bonds of carbon dioxide are at 180° to one another, they oppose each other and cancel out. Carbon dioxide has no molecular dipole and is non-polar. Water also comprises of three atoms but has a different shape. Like carbon dioxide, it has two polar bonds, but, due to its shape they do not oppose each other. They combine to make on side partially positive (where the hydrogen atoms are) and one side partially negative. Water has a molecular dipole and is polar.
The importance of shape on molecular dipoles and polarity
Organic molecules often are harder to judge as they are predominantly based on tetrahedral carbon atoms. All you have to remember is that the four bonds of a tetrahedron will cancel out (if they are the same). This is seen if we compare carbon tetrachloride and chloroform. The former is non-polar as the four polar C–Cl bonds cancel out. The latter is polar due to the hydrogen atom. It is less electronegative than chlorine and this leads to an area of low electron density. There is a molecular dipole.
Comparison of carbon tetrachloride (non-polar) and chloroform (polar)
In larger organic molecules, it is often easier to ‘break’ the molecule into areas or zones. One zone might be polar and one might be non-polar. Then you can judge which zone might have the greater influence on the molecules properties.
Molecules can have polar and non-polar areas
Non-Covalent Interactions
Why is important if a molecule is polar or not? The polarity of a bond influences a molecules reactivity and its physical properties. In this summary, we are concerned with the physical properties and key to understanding these are electrostatic attractions.
Electrostatic attraction means opposite charges attract. These don't have to be full formal charges of +1 or -1 (as found in an ionic bond), simply areas that are electron rich (partially negative) or electron poor (slightly positively charged).
Covalent bonds create molecules, holding atoms together. Non-covalent interactions determine how those molecules interact with each other. These interactions are normally divided into the following categories:
(as always, there should be a little asterisk here that is followed by a statement along the lines of “the summary below is good enough for undergraduate chemistry but there are many other examples of intermolecular interactions such as halogen-bonding, π-stacking etc that are also important'“)
A summary of the common non-covalent interactions (there are more but as an undergraduate this is all you need)
A word of warning regarding terminology - see the note at the bottom of this page!
A common misunderstanding with students concerns the strength of these interactions. Many textbooks provide tables of values showing that ionic bonds are the strongest before listing the rest in descending strength. Such tables show a general trend but often fail to show the overlap between each category. The strength of these interactions will influenced by the shape of the molecules, the size of the molecules, and the separation between the molecules. In other words, the strength can, and will, vary. These interactions are also additive, meaning that a molecule that can cause multiple weak interactions might have a stronger interaction than a molecule that only causes a single example of a strong interaction (table salt dissolves in water). Having said this, when discussing interactions, it is normal to talk about the strongest interaction between two molecules and ignore the rest.
Ionic bonds - the attraction between two charged species, either inorganic ions like those of table salt, Na+ and Cl-, or organic salts such as the ammonium salt of a carboxylic acid, [NH4+][-O2CCH3] or a combination of the two, [Na+] and [-O2CCH3].
Ion-dipole interactions - the electrostatic attraction between a charged species and a neutral molecule with a permanent dipole. This is the most important interaction when discussing the solubility of salts in polar solvents such as water.
Ion-dipole interactions between cation and anion in water
Ion-induced dipole interactions - A weak and momentary attraction between an ion and a non-polar molecule. The ion distorts the electron cloud of the molecule, with cations attracting electrons and anions repelling them. This sets up a temporary dipole in the ‘non-polar' molecule. There is now an attractive interaction between the ion and the now (temporarily) polar molecule. The bigger the molecule's surface area and/or the greater the number of electrons, the more polarisable (easier to distort the electron cloud) the molecule will be.
An ion (the green cation above) can induce a dipole in a non-polar compound that temporarily causes an attraction before dissipating
Van der Waals Interactions
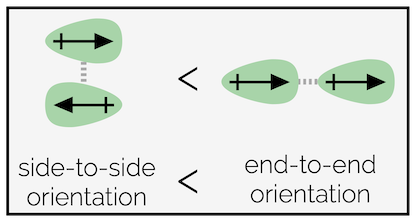
Dipole-dipole (permanent dipole-permanent dipole) interactions - When two polar molecules approach, they can orientate so that the oppositely charged ends attract one another. There are two possible orientations, side-by-side and end-to-end. The latter is favored. This is a strong non-covalent interaction.
Diagram showing the two favored orientations of a dipole-dipole interaction
Hydrogen Bonding - is probably the most important of the non-covalent interactions and is found extensively throughout Nature. Arguably, it is a subset of dipole-dipole interactions but it is normally stronger and it has a directional component. As a result it is normally considered separately.
Hydrogen bonding occurs between a hydrogen bond donor, a hydrogen atom attached to an electronegative atom (H–X), and a hydrogen bond acceptor, a lone pair of electrons on an electronegative atom (X:).
A cartoon of hydrogen bonding and examples showing optimum arrangement of atoms and lone pairs of electrons
A classic hydrogen bond involves either oxygen and/or nitrogen (X = O or N) as this results in the strongest dipole-dipole interaction. The electrons of the H–X bond are polarized towards the electronegative atom leaving a small concentrated partial positive charge on the hydrogen atom. This interacts with the concentrated partial negative charge of a lone pair of electrons on a second row element to give a strong interaction. This interaction is at its strongest when there is a linear arrangement of the X–H bond and the lone pair.
Dipole-induced dipole interactions - In the same way that a charged species can induce a temporary dipole into a non-polar molecule, so can molecules with a permanent dipole. This leads to a momentary or temporary attraction between the two species. It is a weaker interaction than those involving ions.
Permanent dipole–induced dipole interaction with a polar molecule inducing a temporary dipole in a non-polar compound
Induced dipole-induced dipole interactions (or dispersion forces or London forces) - All molecules can interact. The electrons of non-polar molecules are not stationary, and this means that instantaneous dipoles are constantly forming and dissipating. The moment a temporary dipole forms it can induce a dipole in a neighbouring molecule. This causes a momentary attraction between the two molecules. Almost as soon as it forms, it will vanish. These are very weak forces, but they are additive, so become more important the larger the molecule. And, because every molecule can participate in these interactions they are of vital importance. These interactions are between the atoms of two molecules. The more atoms that can get in close proximity the greater the interactions.
Cartoon showing the origin of dispersion forces
Conclusion
This has just been a brief summary of non-covalent interactions or intermolecular forces. These are electrostatic attractions between molecules caused by the uneven distribution of electrons within the molecule. The strongest of these forces are between molecules with a permanent dipole, molecules that have polar bonds. Non-polar molecules can also interact. Either a temporary dipole is induced by a neighboring polar molecule or spontaneous dipoles form (dispersion forces). It is important to remember that these forces are additive, that is bigger molecules or more functionalized molecules can form multiple interactions. The more interactions, the stronger the attraction. It is also important to remember that the strength of these forces are variable.
There are no practice questions attached to this summary but I will add one to the next blog post which will cover this subject (in other words, these two posts were originally one post but it was too long, so I divided it into …)
A Note about Terminology
Common usage changes the meaning of some words. Many synthetic chemists use van der Waals forces as a shorthand for induced dipole-induced dipole (dispersion) forces. Others suggest it means all attractive interactions between non-charged molecules, and still others state that it means all interactions, both repulsive and attractive between non-charged species (there is even an interpretation that states it is all attractive interactions between non-charged species except hydrogen bonding which is its own interaction as it is closer to a covalent bond but that just seems confusing at this level). The terminology is further confused by some people's delight in using the names of the scientists who discovered the forces (van der Waals, London, Debye etc.).
My advice is be specific in your description. If you are discussing induced dipole-induced dipole interactions call it induced dipole-induced dipole interactionst . Don’t call it van der Waals, or London forces. Just describe what you see. This way you will not go wrong.