Nucleophilic Aromatic Substitution
Introduction
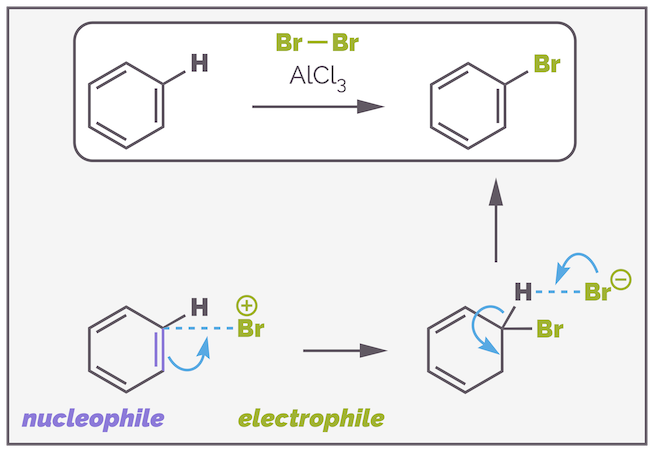
In the previous post, I discussed the nucleophilic properties of aromatic (benzene) rings, and detailed a reaction known as electrophilic aromatic substitution or SEAr. Simplisitically, the ring of π electrons readily attacks a suitably powerful electrophile leading, eventually, to substitution.
Benzene is a reasonable nucleophile and will attack activated electrophiles.
The hydroxide anion HO– will not attack the carbon of bromobenzene* and will not substitute the bromine atom for two reasons. Firstly, the electron rich π system of the aromatic ring repels the anion and secondly, direct substitution is impossible because the anion cannot interact with the C–Br σ* antibonding orbital as it points into the ring.
*Hydroxide will react with bromobenzene under forcing conditions but the mechanism is very different to a direct substitution and will be the topic of a different blog post.
Direct substitution of the bromide with a hydroxide anion is impossible as the aromatic ring is electron rich and repels the nucleophile and because it is impossible for the nucleophile to approach the σ* antibonding orbital due to the presence of the ring.
Nucleophiles will attack an aromatic ring but only if certain criteria are met. The first way to achieve this reaction involves loading the aromatic ring with both electron withdrawing groups and a suitable leaving group. This is known as nucleophilic aromatic substitution and is reminiscent of electrophilic aromatic substitution. The second method involves the use of nitrogen as a leaving group and is more akin to SN1 substitution. Both methods are discussed in this post.
Nucleophilic Aromatic Substitution SNAr
The reaction below shows the substitution of a chlorine atom by hydroxide:
Nucleophilic aromatic substitution involving hydroxide anion attacking the electrophilic aryl chloride. The substitution occurs by an addition-elimination mechanism. In this example an extra neutralization step is necessary as the phenol will be deprotonated by the nucleophile (base).
The reaction is not a direct substitution but an addition-elimination process. The mechanism mirrors that of electrophilic aromatic substitution, which involved an addition followed by deprotonation (or elimination of a proton to re-form the aromatic ring). The differences between the two reactions is that nucleophilic aromatic substitution involves the addition of an anion and the elimination of a leaving group while electrophilic aromatic substitution involved addition of a cation and elimination of a proton.
The hydroxide anion substitutes the chlorine atom through an addition-elimination mechanism. Step 1 involves the addition of the nucleophile to the electron poor (two electron withdrawing groups) aromatic ring. The electrons flow out of the ring onto the nitro oxygen atoms. Step 2 is an elimination. The electrons flow back into the ring, regenerating the aromatic ring. The extra electrons are removed on a suitable leaving group. In this example, the basic conditions would also lead to deprotonation of the product and it would be necessary to neutralize the reaction to get the desired product.
Step 1 of the reaction is the addition of the nucleophile to the aromatic ring (nucleophilic addition). This is the slow step as it breaks the aromaticity of the ring by causing one carbon to become sp3 hybridized. Arguably, there are two carbons that could be attacked by the hydroxide anion, the first is adjacent to the nitro group and the second is adjacent to the chlorine atom. Both carbon atoms would be polarized by the strongly electron withdrawing groups attached to them. The nucleophile attacks the carbon bearing the chlorine atom as this allows the electrons to be delocalized outside of the ring and onto the oxygen atoms. This spreads the charge over a larger area and is more favorable.
In Step 2, the electrons flow back into the ring and the 'extra' electrons are removed when the leaving group is eliminated. This restores the aromaticity. It is a fast and favorable process. In this example, there is an extra step caused by protic nucleophile. The product is a phenol. These are weakly acidic. The reaction involves hydroxide, a strong base, which means the phenol will be deprotonated to create a phenoxide ion. At the end of the reaction, if you want a neutral species, it is necessary to add acid to quench this anion.
General Reaction
Nucleophilic aromatic substitution is a general reaction as long as three criteria are met.
The aromatic ring must activated by a strong electron withdrawing group.
There must be a good leaving group on the ring so that additional electrons of the nucleophile can be removed.
The electron withdrawing group and the leaving group must be either ortho or para to one another. It must be possible for the electrons to flow out of the ring and onto the electron withdrawing group.
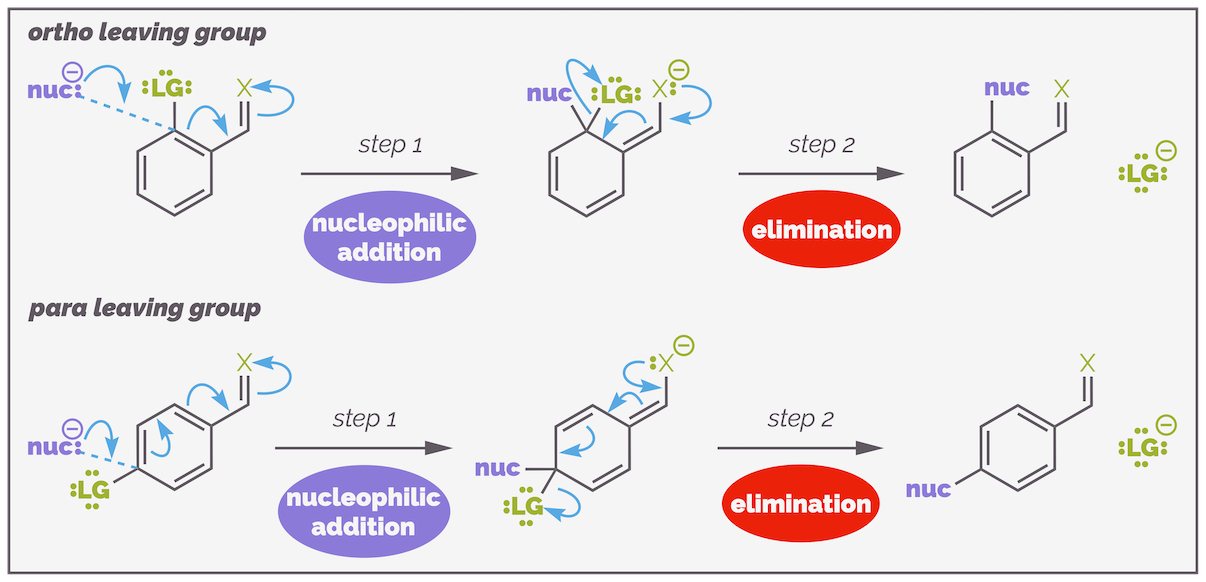
The mechanism can be generalized as shown in the diagram below. After that I'll go through the necessity of the criteria listed above.
The mechanism of nucleophilic aromatic substitution. This is a two step process. Step 1 is nucleophilic addition with the nucleophile attacking the electron deficient aromatic ring. This ring invariably has at least two electron withdrawing substituents. The electrons must flow out of the ring and onto one of the electron withdrawing groups. Step 2 is elimination. The electrons flow back into the ring and onto the leaving group that can then take them away.
For nucleophilic aromatic substitution to be possible the aromatic ring must be activated by the addition of a strong electron withdrawing group. Normally, you should think of an aromatic ring being electron rich, with the π electrons making a good nucleophile (see HERE for electrophilic aromatic substitution). To reverse this, the electrons must be pulled out of the ring.
Addition of the nucleophile to the ring is the slow, rate determining step, as it leads to breaking the aromaticity of the ring with the lose of aromatic stability. This is disfavored. When addition occurs a resonance stabilized, anionic intermediate is formed. This is sometimes known as the Meisenheimer complex. It is the negatively charged analogue of the cationic intermediate found in electrophilic aromatic substitution. I hope the similarities between these two reactions are apparent.
The first step of nucleophilic aromatic addition is addition of the nucleophile to the an electron poor aromatic ring. This step adds two electrons to the molecule and breaks the aromaticity. This is energetically disfavored as there is a lose in aromatic stabilization. The resulting anion is stabilized by resonance and is sometimes called the Meisenheimer complex. This is the anionic equivalent of the cationic intermediate in electrophilic aromatic substitution.
This electron withdrawing group must be conjugated* with the ring to allow the electrons to flow out of the ring. The most common, and probably best, group is the nitro group but you can also use nitriles, carbonyl containing groups and sulfones as in the example below. All of these groups permit electrons to flow out of the ring and ‘sit’ on an electronegative element prior to the elimination step.
*Alright, this is not entirely true, –CF3 will work as well but this is due to the strong electron withdrawing effect of three highly electronegative fluorine atoms. And while it works, the reactions are considerably slower than those that allow the electrons to be delocalized outside the ring.
The similarities between nucleophilic aromatic substitution and electrophilic aromatic substitution should be apparent. The first step involves nucleophilic addition either of a nucleophile to the ring or of the ring to a suitable electrophile. This step destroys the aromaticity so is slow and disfavored. The resulting anionic or cationic intermediate is stabilized by resonance but is still higher in energy than an aromatic or fully delocalized system. The next step will be similar for both ...
The next example shows a sulfone acting as the electron withdrawing group. The mechanism is identical to that for the nitro group. The only difference is found in the real world (and not on a computer screen), the reaction is slower than the analogous reaction with a nitro group. But on paper, there is no difference with the curly arrows you draw.
Sulfones are good electron withdrawing groups for nucleophilic aromatic substitution. The amine attacks the electron poor ring and the electrons flow out of the aromatic ring and onto the electronegative oxygen atom of the sulfone. In the last step the electrons flow back into the ring and restore aromaticity.
The next factor necessary for nucleophilic aromatic substitution is a leaving group. When the nucleophile attacks the aromatic ring it adds two electrons and this leads to a negative charge being stabilized by the electron withdrawing group. But, at some point, two electrons must be removed if you are to get a stable, neutral compound back. The two electrons are removed by a leaving group.
The leaving group is normally a halide, and every example above uses a halide. These are electronegative allowing them to stabilize the anion (they are weak bases or have a strong conjugate acid). Other groups that stabilize an anion will behave as leaving groups such as sulfonate esters.
A sulfone can be a leaving group in electrophilic aromatic substitution. It is slightly electron withdrawing (which activates the ring) and forms a resonance stabilized conjugate base (stable anion).
Unusually, fluorides are excellent in these substitution reactions. Normally fluorides are poor substrates for substitution reactions as the C–F bond is the strongest single bond to carbon. But in this case, the extreme electronegativity of fluorine helps activate the ring to nucleophilic addition by making it more electron deficient, this accelerates the first step (the rate determining, aromaticity breaking first step). The second step kicks out the fluoride which can stabilize an anion so will leave. In fact, fluorides turn out to be the best substrates in these reactions.
The electronegative fluorine activates the ring to nucleophilic addition in step 1. It makes the ring more electron deficient. It is then ok to kick it out in the second step as it can stabilize an anion.
Finally, for nucleophilic aromatic substitution, the leaving group and the electron withdrawing activating group must be in the correct positions. They most be either ortho or para to each other. If they are not, then the electrons of the nucleophile will be unable to flow out of the ring and the reaction cannot occur.
The leaving group and the electron withdrawing activating group must be either ortho or para to each other. This pattern allows the incoming electrons of the nucleophile to be pushed out onto the activating group giving the anionic intermediate extra stability. If the substituents are meta, then the electrons are only stabilized within the ring, which is less favorable.
Attack at any position allows the anionic intermediate to be delocalized to three positions on the ring but only attack at the ortho or para positions permits the anionic intermediate or Meisenheimer intermediate to be stabilized outside the ring. These extra resonance structures are shown in green on the diagram above. The afford extra stability and permit the reaction to proceed.
Overall, the reaction requires an activated electrophile, the aromatic ring must be electron deficient with an electron-withdrawing group that permits the electrons to flow out of the ring. There must be another electron withdrawing group that can be eliminated and this must be in the ortho or para positions. While I haven't discussed the nucleophile, these are invariably either nitrogen, oxygen or sulfur-based nucleophiles or the cyanide anion. The example below shows the synthesis of 2,4-dinitrophenylhydrazine or DNP.
2,4-Dinitrophenylhydrazine or DNP can be synthesized by nucleophilic aromatic substitution. Step 1 involves the nucleophilic addition of hydrazine to an aryl chloride leading to an anionic intermediate with the negative charge stabilized outside the ring. After a proton transfer, step 2 is the elimination, where the electrons flow back into the ring and kick out the chloride anion. The process is addition and elimination.
Nucleophilic aromatic substitution using diazonium chemistry (an SN1-like mechanism or aromatic SN1)
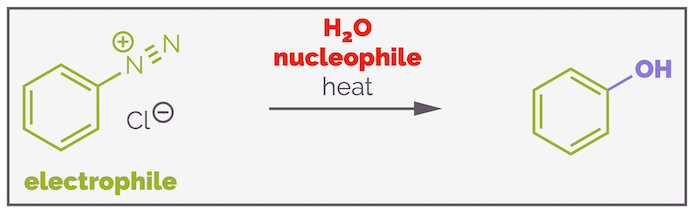
There is another way to achieve nucleophilic aromatic substitution and it does not require the benzene ring to be substituted with electron withdrawing groups. Instead, all you need is a very good leaving group in the shape of nitrogen gas. Reacting a diazonium salt in the presence of a nucleophile leads to substitution (amongst other reactions).
The formation of phenol from a diazonium salt by nucleophilic aromatic substitution.
The majority of diazonium salts are not very stable. It should be apparent that that they can easily eliminate nitrogen gas. In this blog post that is the whole point of them! I’m sure in a separate post I’ll discuss the synthesis of azo dyes but not here. The high reactivity means they need to be synthesized directly before the substitution reaction. The textbook reaction for their preparation is sodium nitrite and a mineral acid, although there are other reagents, such as amyl nitrite, that can achieve the same transformation under milder conditions.
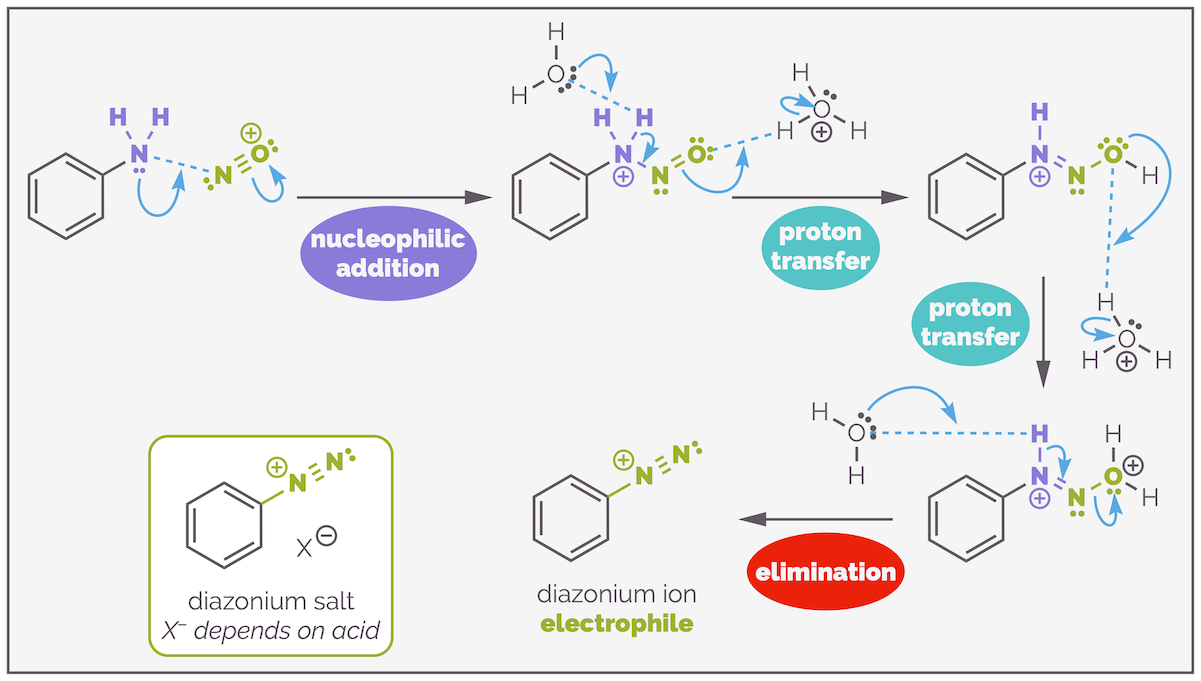
The mechanism for diazotization is long but relatively straightforward (especially if you have already looked at electrophilic aromatic substitution. The mechanism of diazotization shares many similarities with nitration as there is a series of dehydrations that result in a highly electrophilic, cationic species). The first stage is to convert sodium nitrite into the nitrosonium ion NO+.
Formation of the powerful electrophile, the nitrosonium ion. The nitrite anion undergoes a series of protonations, first to give nitrous acid, then a cationic species that loses water to give the nitrosonium ion.
Nitrosonium formation starts with two protonations. The first converts the nitrite anion to nitrous acid and the second forms a good leaving group in the formation of the oxonium ion (water as the leaving group). Water is kicked out in an elimination that forms the nitrosonium ion. This is a powerful electrophile that can be drawn as two resonance structures.
The nitrosonium ion reacts with a primary aromatic amine. Nucleophilic addition of the amine to the cationic species is followed by a series of proton transfers that create another oxonium ion leaving group. Again, water is eliminated in a dehydration reaction that forms the diazonium ion.
The mechanism for diazotization or formation of the diazonium salt looks long but is relatively straight forward and involves reaction steps you have seen many times before. The first step is nucleophilic addition to the activated electrophile, the nitrosonium ion. There are then a series of proton transfers that create an oxonium ion. Finally, this is eliminated to give thee diazonium species.
Diazonium salts readily decompose to give nitrogen gas and a carbocation or aryl cation. A cation on an sp2 hybridized carbon is very reactive. There is no hyperconjugation (σ conjugation) causing stabilization. A nucleophile can then attack the empty sp2 orbital. After a proton transfer you have the product.*
*As you will soon see, this is a simplification. The mechanism for the addition of some nucleophiles involves an aryl radical. But this is good enough to start with.
Effectively, you have reversed the steps of the previous substitution. Instead of addition followed by elimination, the substitution of an aryl diazonium salt proceeds by elimination-addition mechanism.
The mechanism of nucleophilic aromatic substitution of diazonium compound involves the elimination of nitrogen gas to give an aryl cation. This is a powerful electrophile which is rapidly attacked by a nucleophile. Proton transfer (in this case) then gives the product.
A variety of nucleophiles can be use in the reaction. The most common are given below and include water, all of the halides and cyanide. When the reactions involve copper(I) salts they are often known as Sandmeyer reactions and occur through a radical reaction mechanism.
Diazonium salts readily loss nitrogen to give a cation that can then react with the appropriate nucleophile to give nucleophilic aromatic substitution. If the nucleophile is a copper(I) reagent then the reaction is probably related to the Sandmeyer reaction and proceeds by a radical mechanism.
The mechanism of the Sandmeyer reaction is beyond the scope of most undergraduate courses. The copper(I) salt almost certainly acts as a reductant, giving an electron to the diazonium ion, and becoming a copper(II) salt. The neutral radical collapses to an aryl radical and nitrogen gas. The next bit is magic. The radical could either abstract the nucleophile from the copper(II) salt, leading to product and another copper(I) salt, or it could add to the copper(II) species itself to give an organocopper species that undergoes reductive elimination to give the product and a copper(I) salt.
The first diagram shows the direct abstraction of the ‘nucleophile. The second shows the trapping of an organocopper species and reductive elimination.
One mechanism for a Sandmeyer-like reaction involves the copper(I) salt reducing the diazonium salt. This causes the copper(I) to be oxidised to copper(II). The copper(II) species probably picks up a counterion. The neutral diazo radical undergoes an elimination to give the aryl radical. This adds directly to the copper(II) species to add the ‘nucleophile’ and regenerate copper(I).
A second potential mechanism starts in the same manner. Once the aryl radical is formed, it adds to the copper salt to give a copper(III) species or organocuprate. This undergoes reductive elimination to give the product.
The use of diazonium salts demonstrates the usefulness of nitrogen-containing functional groups in the synthesis of aromatic compounds. Simple nitration (see electrophilic aromatic substitution HERE) introduces nitrogen to a benzene ring. The nitro group potentially activates the ring to nucleophilic aromatic substitution (see above) of a suitable leaving group. Alternatively, the nitro group can be reduced to an amine. This activates the ring towards electrophilic aromatic substitution or can be converted to the diazonium species to allow direct substitution of nitrogen. The example below is from the synthesis of fluanxol.
An example of the Sandmeyer reaction being used in the synthesis of a pharmaceutical.
Conclusion
The post looks at two different approaches to nucleophilic aromatic substitution (arguably three approaches as formation of a cation or radical is different). The first version of nucleophilic aromatic substitution proceeds by an addition-elimination mechanism. It requires the aromatic ring to be activated with electron withdrawing groups that make the ring electron deficient and prone to attack by a nucleophile. The nucleophile will attack the carbon atom that has a leaving group attached. This leaving group must be capable of taking two electrons away and is normally a halide, with fluoride being the most reactive. When the nucleophile attacks, the electrons flow towards the activating group and should be stabilized outside the ring. This means the activating group and the leaving group must be ortho or para to each other. In these reactions, the nucleophile is normally nitrogen or oxygen based or the cyanide anion.
The second nucleophilic aromatic substitution is more general. There is no requirement for any other group being attached to the aromatic ring other than a nitrogen atom. The nitrogen is converted into the ultimate leaving group in the form of a diazonium salt. This is known as diazotization. Once formed the diazonium salt can eliminate nitrogen gas to give a cation or an aryl radical. These can be attacked by a range of nucleophiles and permit phenols, aryl halides and benzonitriles to be prepared.
There is one more nucleophilic aromatic substitution reaction that I’ll discuss and that is the benzyne mechanism.