Predicting the relative strengths of acids (or bases)
Or, can I predict which molecule will be the stronger acid just by looking at their structure?
Introduction
The quick answer to this question is "yes", the long answer is the rest of this summary.
In a previous summary (HERE), I showed how pKa values are used to indicate an acid's strength. Compounds with a low pKa are acidic, and readily lose a proton to a suitable base. Triflic acid, with a pKa of -14 will fully dissociated in the presence of a base (there is no TfOH in solution only TfO- and HB+), while ammonia, pKa = 38, is hard to deprotonate and is a very weak acid. But there will be times when you will not have access to a pKa table, or the compounds you are studying may not be on a pKa table. You need to be able to assess which of two compounds is likely to be the most acidic just by comparing their structures). Ultimately, this is a valuable skill that will make the study of reactions far easier.
Given two molecules or functional groups, where do you start when trying to assess their acidity? The best practice for a chemist is to draw the reaction and look at the species involved. The general reaction of an acid is:
The general reaction of an acid and a base.
A strong acid favors the right-hand side of the reaction with dissociation of A–H giving the conjugate base A-. A strong acid HA will have a stable conjugate base A-. The factors that influence the stability of the anion influence the strength of the acid and we will look at each in turn.
While this summary focusses on the structural features that influence the strength of an acid, all the principles covered can be used to predict basicity. Just remember that the strength of a base can also be assessed by the pKa of its conjugate acid BH+, or what is sometimes known as pKaH. The higher the pKaH the weaker the conjugate acid and the stronger the base. To apply the same concepts as this summary means looking at the factors the influence the 'stability' or lack of reactivity of the lone pair of electrons (how easy is it to donate the lone pair to make a covalent bond?). This sometimes confuses students (the lone pair is stable, the Octet rule tells us it must be there) but if you remember that a negative charge in organic molecules is a lone pairs of electrons (but not all formal charges are). So, if you want to assess the 'stability' of a lone pair just think of it as a charge (an anion) and use the summary below. This will lead you to the correct answer (most of the time ...).
What Factors Influence Acidity or the Stability of the Conjugate Base A-?
The more acidic a proton is, the more stable the resulting conjugate base. The key factors effecting the stability of A- are:
1. Which atom the charge is on - both electronegativity and size affect stability.
2. Delocalization - can the charge be spread over multiple atoms?
3. Inductive effect - electron withdrawing groups close to the charge will increase its stability.
4. Hybridization of the atom - changing hybridization changes stability.
5. Other factors such as solvents can influence the stability of an anion but will not be discussed in much detail
Factors influencing the stability of the conjugate base A-.
1. What element is the charge on?
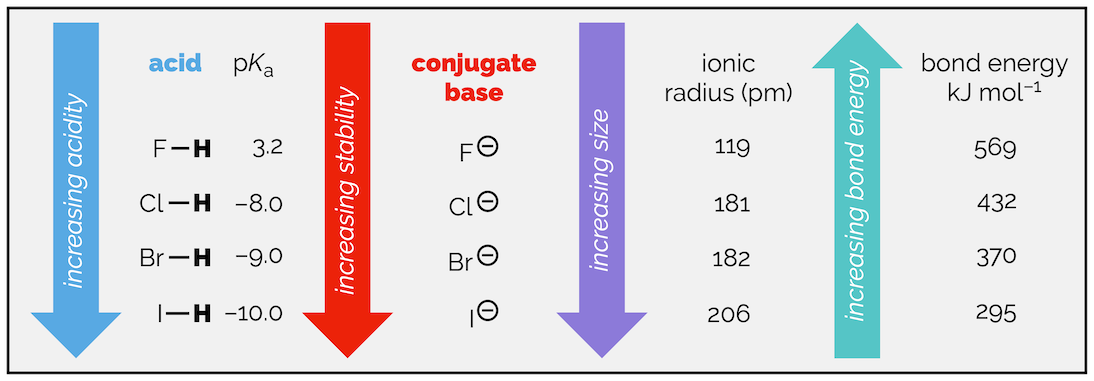
The most important factor to the stability of a conjugate base is which element the charge is on, with both the electronegativity of the atom and its size playing a role. Electronegativity is key when comparing elements in the same row of the periodic table. Acidity increases going from carbon to fluorine, in line with the increase in electronegativity. This is seen if you compare the acidity of methane, ammonia, water and hydrofluoric acid.
Effect of electronegativity on the stability of the conjugate acid as you move across a row of the periodic table.
While electronegativity is important when comparing elements in the same row of the periodic table it is not if you are looking at elements in the same group. As you move down a group, electronegativity decreases yet the stability of the conjugate base and the acidity increases. There are two arguments for this observation but they effectively amount to the same thing, as the size of the element increases so the stability of the anion increases and the acid will be more acidic. Alternatively, as the atom gets bigger, the bond to hydrogen gets weaker and the acid becomes stronger.
Effect of the size of an ion or the strength of the bond on acidity.
In terms of this summary, it is more consistent to think of the acidity increasing as stability of the conjugate base or anion increases when you move down a group. The larger the anion, the greater the volume of space the charge is spread over and the more stable it is.
You should be aware though that bond strength is also changing. The size of the element effects the strength of the bond between the atom and hydrogen. The larger the atom, the weaker the bond to hydrogen. As the atom gets bigger, so the overlap between the (hybrid) atomic orbital and the 1s atomic orbital of hydrogen becomes less and the bond is more readily broken. Or the compound is more acidic.
A cartoon representing poor overlap. The bigger the difference in size of atoms the weaker the bond connecting them.
Example of ranking acidity
Just using the first criterion, it is possible to rank the three compounds below from most to least acidic without looking up their pKa values.
To determine which compound is the most acidic, you should start by determining which conjugate base is will be the most stable. It is not possible to compare all three compounds directly as the thiol (t-BuSH) and the amine (t-BuNH2) are not in either the same row or group of the periodic table. It is possible to compare them both to the alcohol (t-BuOH) and from this indirectly determine the order.
Comparing the thiol to the alcohol, the conjugate base has the charge on either a sulfur atom or an oxygen atom. Both elements are in Group 16 and you should consider their size when thinking about stability. Sulfur is larger than oxygen, or further down the group. This means it can stabilize the charge over a larger volume and/or the S–H bond is weaker than the O–H bond. The thiol is the more acidic compound.
By comparing the thiol to the alcohol and the alcohol to the amine, it is possible to determine the order of acidity.
Next compare the alcohol to the amine. These two have the charge of the conjugate base on oxygen and nitrogen. The elements are in the same row of the periodic table so electronegativity is important. Electronegativity increases as you move left to right so the oxygen is more electronegative than nitrogen. The alkoxide is more stable than the amide, meaning the alcohol is more acidic than the amine.
You now know that the thiol is more acidic than the alcohol, which is more acidic than the amine. There is no need to compare the thiol to the amine. The order of acidity is shown pictorially below along with approximate pKa values just to try and convince you that the method works!
The correct order of the compounds along with approximate pKa values (for most similar compounds).
2. Delocalization of charge
The negative charge of a conjugate base can be stabilized by spreading it over multiple atoms. This occurs when it is possible to draw multiple resonance structures for the anion or the anion is said to be delocalized over multiple atoms. The effect of delocalization can be seen when comparing the acidity of the four mineral acids below:
The effect of increasing delocalization of charge in the conjugate base.
For the four acids above, the charge is always on an oxygen atom, so neither electronegativity nor size can differentiate the acids. The difference is the number of atoms the charge is shared or delocalized over. For hypochlorous acid, the charge is localized on a single oxygen atom. For perchloric acid, the charge is delocalized over four atoms. Spreading the charge over more atoms results in a more stable conjugate base and hence a stronger acid.
The same reasoning can be applied to the difference between an alcohol and a carboxylic acid. Ethanol is less acidic than acetic acid as the charge on the conjugate base is localized on a single oxygen while in the carboxylate anion it is delocalized over two atoms.
* this is the pKa for methanol, the pKa of ethanol will be slightly higher but probably not much (see inductive effect).
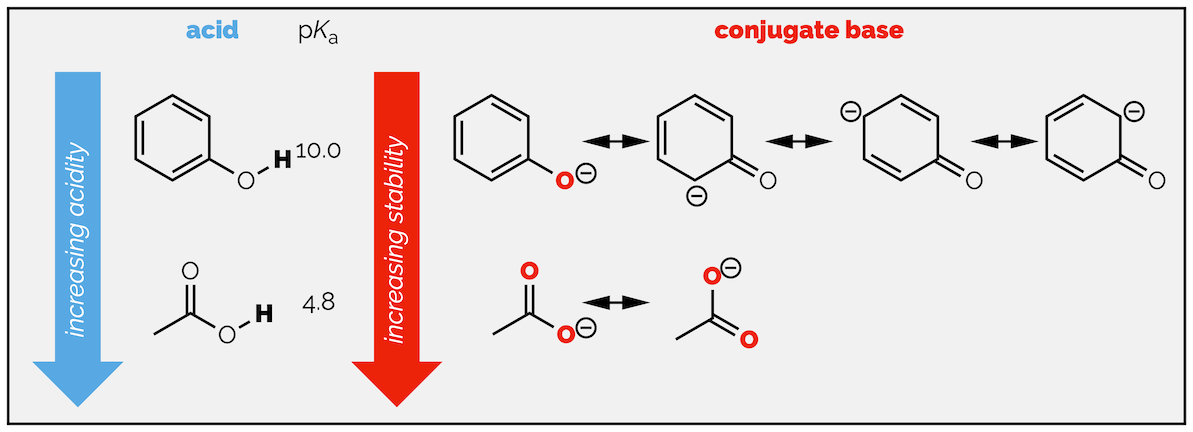
Generally speaking, the more spread out the charge is, the more stable it is going to be. This can be interpreted as the more resonance structures you can draw, the more stable the anion. But you must be careful, the first factor, which atom the charge is on is still important. This means that not all resonance structures are created equal. A resonance structure that places the charge on an electronegative atom is more important than one (or more) that don't. This is one (of two) arguments that can be used to explain why acetic acid is more acidic than phenol (the second reason is the inductive effect).
Delocalization in phenol and acetic acid. Spreading the charge over electronegative oxygen atoms has a greater effect than spreading the charge of multiple carbon atoms (and the inductive effect described later is also important).
This tells you that it delocalization over two electronegative oxygen atoms causes more stabilization of the charge than spreading it over the aromatic ring. Some textbooks fail to mention that this isn’t the only reason acetic acid is more acidic than phenol. Doing so it problematic as it can lead students to make a wrong prediction about the acidity of substituted phenols.
The key is to remember that you should consider all four factors that influence the stability of the conjugate base before arriving at a decision. To look at just one factor can lead to errors. It is also worth realizing that the concepts outlined in these summaries are not hard and fast rules but are a launch pad to enable you to start thinking like a chemist and thinking about the interplay of the various factors that can influence acidity. Then next example hopefully gives you more insight …
It is possible to to increase the acidity of phenol by extending conjugation and delocalizing the charge onto a second oxygen atom, yet this still does not make the phenol more acidic than acetic acid.
Increased delocalization lowers the pKa of phenol but not by as much as you might think.
The nitrophenol still isn't as acidic as acetic acid because resonance is only one of the factors influencing the stability of the negative charge. There is also an inductive effect (see below) that is distance dependent, the anion on the acid is directly attached to a polarized electron withdrawing group, the carbonyl group. This has a big impact. By thinking of each factor in isolation, you will end up with the wrong answer. Again, I stress, I'm not trying to provide definitive rules for predicting exact values of acidity but rather presenting a framework to start thinking about the chemistry of a molecule.
The position of the nitro group on the benzene ring is important. Resonance stabilization is only possible if the nitro group is in the ortho or para positions. Both these phenols are more acidic than the meta-isomer.
Resonance stabilization is only possible if the nitro group is ortho or para. It is not possible with a meta substituent.
Example of ranking acidity using delocalization
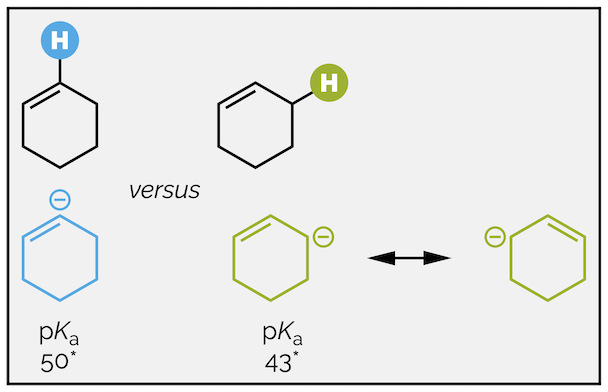
Which of the two protons highlighted on the following molecule is the most acidic?
To predict the relative acidities you again should inspect the stability of the two difference conjugate bases (this is a good rule!). In both cases the anion is on a carbon atom so you cannot use electronegativity or size to differentiate them. The conjugate base formed from deprotonation of the green hydrogen (an allylic hydrogen - yeah, chemists love giving things names) can be delocalized while the anion from removal of the blue hydrogen (a vinylic hydrogen) cannot. The green anion is more stable and the green proton is more acidic.
The allylic proton is more acidic due to delocalization. * approximate pKa values for similar molecules.
3. The Inductive Effect
Electron withdrawing groups close to the charge of the conjugate base lead to greater stability and thus greater acidity (conversely, electron donating groups will destablize the charge). This is clearly seen if you compare the acidity of acetic acid with the fluorinated derivatives below. Each additional electronegative fluorine atom stabilizes the negative charge more by increasing the partial positive δ+ charge on the carbon atom.
The inductive effect explains the increasing acidity of fluoroacetic acid derivatives compared to acetic acid.
If the fluorine atoms were replaced by methyl groups, giving you pivalic acid (trimethylacetic acid), then the compound would be less acidic. Alkyl groups are electron donating due to hyperconjugation and this destablizes the charge on the conjugate base. The pKa of pivalic acid is 4.9 or 5.0 (acetic acid is 4.8).
The inductive effect shows a strong distance dependency with its influence rapidly dropping off the further the electronegative group is from the negative charge. If you compare the three isomers of chlorophenol you see that the acidity drops as the electronegative chlorine atom moves further from the oxygen (ortho to meta to para positions).
Proximity of an electronegative element, or electron withdrawing group, effects the acidity of the phenol.
Electron donating groups have the opposite effect, they destabilize the conjugate base, making the acid weaker. This isn't normally important when you are looking at the acidity of compounds but can be when you consider how basic a molecule is. Remember that a strong base will have a weak conjugate acid or an a conjugate acid with a high pKa value. Ammonia is a weaker base than triethylamine as the conjugate acid, the ammonium cation, has a lower pKa than triethylammonium cation. The three alkyl groups are electron donating and 'destablize' (a horrible phrase, I guess I should sya they make it more reactive but I want some consistency in this summary so I'm trying to make it about stabilization) the nitrogen lone pair making the conjugate acid more stable and less acidic. Or triethylamine more basic.
The inductive effect can destabilize a base and make the compound more basic (or the conjugate acid less acidic).
Example of ranking acidity using the inductive effect
Using the idea of the inductive effect, it should be relatively easy to predict which of the two labelled protons is the most acidic.
To determine which proton is the most acidic, compare the stability of the two different conjugate bases. One has the alkoxide in close proximity to two electronegative fluorine atoms. The electron withdrawing inductive effect stabilizes the anion more. The blue proton is more acidic.
4. The Effect of Atom Hybridization
Alkynes are a useful functional group that can be converted into nucleophiles by deprotonation. Why is it easier to deprotonate an alkyne than either an alkene or alkane? Why are alkynes more acidic?
Why is the proton on an alkyne more acid than that of either an alkene or alkane?
The difference in acidity cannot be predicted using the first three factors. The conjugate base would have the anion on a carbon atom in all three examples. There is no delocalization of the conjugate base and there is no inductive effect. Yet the alkyne is more acidic. The difference is the hybridization of the carbon atoms.
Effect of hybridization on stability of conjugate base.
As always, when predicting the relative acidity, you look at the the stability of the conjugate base. In each case the anion is on a carbon atom but the lone pair of electrons that makes up the anion is part of a different hybrid atomic orbital (is in a different orbital? Or is a different orbital?). The alkyne is sp, the alkene is sp2 and alkane is sp3. The hybrid molecular orbitals are not the same. In most undergraduate courses, students are taught that the orbitals only differ in the direction they are orientated but it is often glossed over that they have slightly different sizes and shapes. The reason for this is that they are ‘made’ from different combinations of atomic orbitals. An s orbital is a sphere while a p orbital is a long dumbbell. The more s character that goes into making a hybrid atomic orbital, the smaller and more spherical it will be. Electrons will be closer to the nucleus of an atom and the attractive positive force of the protons. They are more stable. The more p character in a hybrid orbital and the larger it will be (larger means more energy). The further the electrons can be from the nucleus, the more energy they will have and the less stable.
A negative charge on an sp hybridized atom, which has 50% s character, will be more stable than a negative charge on an sp2 hybridized atom, which has only 33% s character. Both will be more stable that a negative charge on an sp3 atom, which is a mere 25% s. A proton on an sp hybridized atom is more acidic than a proton on an sp2 hybridized atom, which is more acidic than a proton on an sp3 hybridized atom.
A little dirty secret that lecturers never tell undergraduate students is that the hybridization of an atom can be influence by the shape of the molecule. So the carbon of cyclopropane aren't sp3 hybridized (apparently, they are considered sp5 ... don't ask) and this will effect acidity (of course, the dirtiest secret is that hybridization isn't real ...).
Example
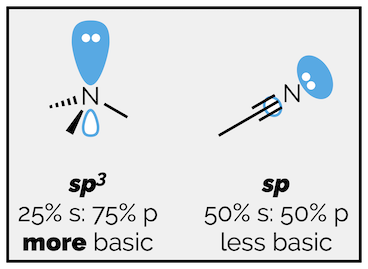
Hybridization will effect basicity as well (for exactly the same reason). You should be able to predict whether trimethylamine is more or less basic than acetonitrile based on hybridization.
The lone pair of electrons on trimethylamine is on an sp3 hybridized atom. The lone pair on acetonitrile is on a sp hybridized nitrogen atom. The sp hybridized orbital is 50% s character. It is closer to the nitrogen atom and the electrons are held more closely by the positive nucleus. These electrons are less available for protonation. Acetonitrile is less basic than trimethylamine.
5. Other Factors
I’m not going to discuss other factors in detail. The idea is to look at the basics, but you need to be aware that other factors play a role (and that these aren’t rules, there will be exceptions).
Solvent plays a very important role in determining acidity. Some solvents are better at stabilising the conjugate base than others. Molecules will dissociate more readily in such solvents and will behave as strong acids. For this reason, pKa values should always be quoted with the solvent they were determined in and you cannot compare pKa values of two compounds taken in two different solvents (without using a conversion factor).
Solvent will influence the ease of dissociation or deprotonation.
Polar solvents and protic solvents stabilize the conjugate base better by effectively allowing the charge to spread over a greater volume. Acids will dissociate more readily in polar solvents.
The steric bulk or size of a molecule can influence acidity (and basicity). if the bulk of the molecule prevents solvent approaching the conjugate base then the molecule loses the stabilizing influence of the solvent. The conjugate base is less stable. The molecule will be less acidic (or more basic).
6. Prioritizing the factors
Which of the four factors is most important when predicting acidity, the atom, delocalization, the inductive effect or hybridization? The atom the charge is on is normally the most important and I have listed the factors in the general order of importance.
Atom
Delocalization
Inductive effect
Hybridization
But I cannot stress enough that these are not rules. They are just a series of guidelines to help you think about what influences acidity. There will be exceptions. There will be exceptions because all the factors (and others) are important at the same time. You should always consider all factors before coming to a conclusion.
The most common exception is the use of sodium amide to deprotonate an alkyne. This is a classic reaction, and it doesn’t match the guidelines outlined above.
Sodium amide is a sufficiently strong base to deprotonate an alkyne.
Using the guidelines above, ammonia should be the stronger acid than an alkyne. Its conjugate base has the charge on the electronegative nitrogen atom while an alkyne is on a carbon atom. In this case, the hybridization of the carbon atom, sp compared to sp3 for the nitrogen, is more important. The alkyne anion is more stable the amide so it is more acidic. Or the amide is sufficiently basic to deprotonate the alkyne.
The guidelines are only here to help you think about the chemistry. They will be very helpful but they will not always be correct.
Why do we want to predict acidity?
As stated in the introduction, it is a useful skill especially as the pKa values are not known for all compounds. Being able to predict the stability of the conjugate base is helpful when looking at reactions. It will be useful for predicting leaving groups when we start looking at acyl substitution and substitution on saturated alkyl groups. It can also give you an idea as to which side of an equilibrium is favoured (just not by how much).
General acid base reaction.
Consider the general reaction above, both sides of the equation contain a base (or conjugate base). You are now in a position to assess which (conjugate) base would be more stable. The reaction will favor this side of the equation. Reactions favor the more stable, less reactive product, or the side of the reaction with the weaker (conjugate) base and the weaker (conjugate) acid.
For example, mixing benzoic acid and sodium hydroxide sets up the following equilibrium:
An example of an acid base reaction.
To predict whether the reaction favors the left- or right-hand side you should compare the stability of the two anions. First, consider which atom the charge is on. In both cases it is an oxygen atom so there is no difference. Next look for delocalization. On the left-hand side, the anion is localized on a single oxygen atom while on the right-hand side it is delocalized over two oxygen atoms. The carboxylate anion is more stable than the hydroxide anion. The third factor is the inductive effect. The hydroxide shows no inductive effect but the carboxylate anion on the right-hand side has the negative charge next to a highly polarized carbonyl group. It is stablized by an inductive effect. Finally, hybridization. Some would argue that both are sp3 oxygen atoms. I favor the interpretation that both carboxylate oxygen atoms must be sp2 to allow delocalization. This means two of the lone pairs of the oxygen are in sp2 hybrid orbitals and are closer to the nucleus so are stabilized. Three factors suggest that the carboxylate anion is more stable than the hydroxide anion. The reaction favors the right-hand side or products.
Conclusion
The relative acidity of two, or more, molecules can be predicted by assessing the factors that influence the stability of each of the conjugate bases. The main factors are listed below in the order of importance:
- Which atom the charge is on - compare the electronegativity and the size.
- If the charge is delocalized - can the charge by spread out (preferably onto electronegative atoms).
- If functionality than can cause an inductive effect is close by - electron withdrawing groups stabilize an anion while electron donating groups can destabilize the charge.
- The hybridization of the atom - a negative charge on an sp hybridized atom is more stable than those on sp2 or sp3 atoms.
You must consider all factors and there are exceptions to the order of this list. It is simply a set of guidelines that aid your thought process, but time and experience will give you a better feel for acidity (and if you aren’t planning on becoming a chemist and just need to pass a chemistry paper then they will undoubtedly hold true for the examples you are given).