An Introduction to Stereochemistry
Introduction
The shape of a molecule influences physical and chemical properties. The shape of a molecule is more complex than just looking at each atom within it. An alkene with its two sp2 hybridized, trigonal planar carbon atoms can exist as two possible shapes depending on the groups attached. It can be cis or trans to the on whether the groups are on the same side or opposite sides. The molecule could be curled in a ball or an elongated zig-zag. Similar issues occur with sp3 or tetrahedral carbon atoms, where it is possible to have two almost identical molecules that are mirror images and differ in the same way our left and right hands differ. This is stereoisomerism.
Below is an introduction to this important topic, a topic that can rapidly become very complicated (as anyone who has read the big red Eliel & Wilen will attest to). I have ignored conformations even though they are arguably stereoisomers (especially if you look at atropisomers, molecules with restricted rotation around a single bond). I have chosen to only considered compounds with different configurations. This post is aimed at undergraduates starting their chemical careers (or other scientific endeavours) and not at pedantic post-graduates (or worse, my peers & colleagues). If I keep writing these blogs for long enough, I’ll eventually write a more precise discussion of stereochemistry (which will find all new ways to annoy my colleagues).
Stereoisomers and alkenes
The two alkenes below have an identical constitution - they have the same molecular formula, containing the same atoms. They also have the same connectivity, with the atoms joined in the same order. But they are clearly different molecules with different properties. They are different because they have a different shape.
The two stereoisomers of butenedioic acid
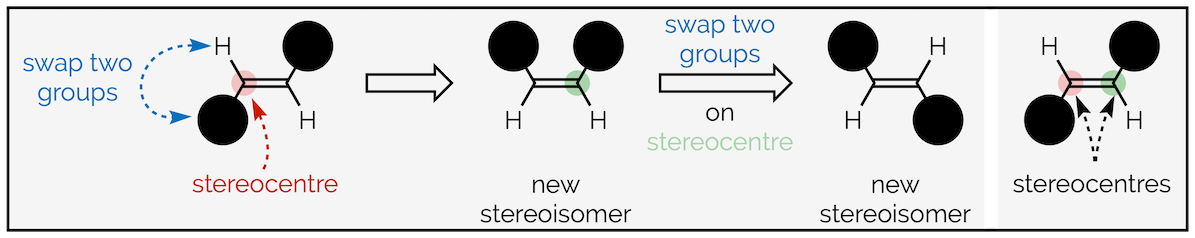
Compounds that only differ by their shape and cannot be interconverted without breaking a bond (I’ve added this, even though it isn’t true so that I can avoid the discussion of conformations, the change in shape caused by rotation of a σ bond) are called stereoisomers. The two stereoisomers differ because of the of the groups of atoms on a stereocentre (this is one of many simplifications. I’m aiming to get the basics across). A stereocentre is an atom which, when you swap two groups attached to that atom, gives you a different stereoisomer. With an alkene, both the carbon atoms are stereocentres.
The two stereocentres of an alkene
Stereochemistry and tetrahedral carbon atoms
The stereoisomers of an alkene are easy to visualize, they are cis and trans isomers. The groups are either on the same side or opposite sides. The stereoisomers of tetrahedral atoms are hard to spot. There are two stereoisomers of alanine, a simple amino acid. These are drawn below in their non-zwitterionic form. Key to understanding that these molecules are different is recognizing their three-dimensional shape, they are tetrahedrons. In the drawings this is depicted by using the bold wedge and the hashed wedged. The bold wedge is pointing upwards, out of the screen towards you. The hashed line is pointing away from the viewer and is considered to be going downwards.
The two stereoisomers (enantiomers) of alanine
A 3D model of D-alanine
Both molecules have the same constitution as indicated by their molecular formula. Both molecules have the same connectivity with each atom joined to the same atoms. But they are different. It is impossible to rotate, flip, twist or manipulate one molecule so that it will occupy the same space as the other. They are non-superposable mirror images like your left and right hands. They have a different shape and are stereoisomers.
The stereocentre is indicated on the diagram (the red circle). If you swap two groups on this carbon atom, for example the nitrogen and hydrogen atoms, you form the other stereoisomer. The absolute arrangement of atoms around the stereocentre is known as the configuration of the atom. The two stereoisomers have different configurations.
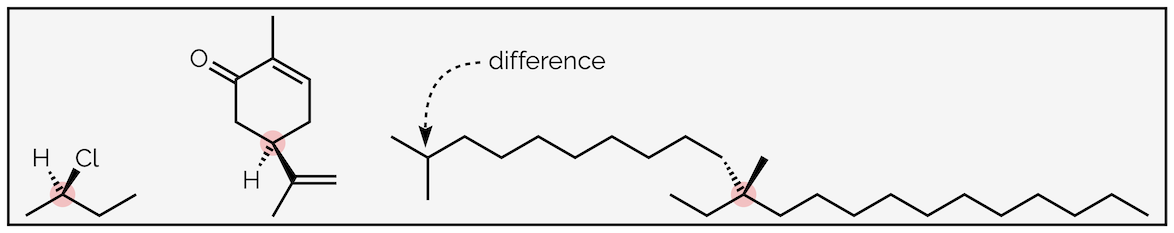
To identify whether a sp3 tetrahedral carbon is a stereocentre you must look at the groups attached to it. If there are four different substituents it is a stereocentre (this is yet another simplification but it holds at first year but if you are curious to see an exception, look up the structure of olean, a pheromone found in olive fruit flies. This only has two ‘different’ groups and yet the molecule exists as two stereoisomers). Three examples are given below. Note that the difference does not have to be on the atom directly attached to the stereocentre, the groups just have to be different.
Three examples of sp3 carbon stereocentres
If any groups attached to an atom are the same then it will not be a stereocentre (at first year - see olean).
Chiral and achiral molecules
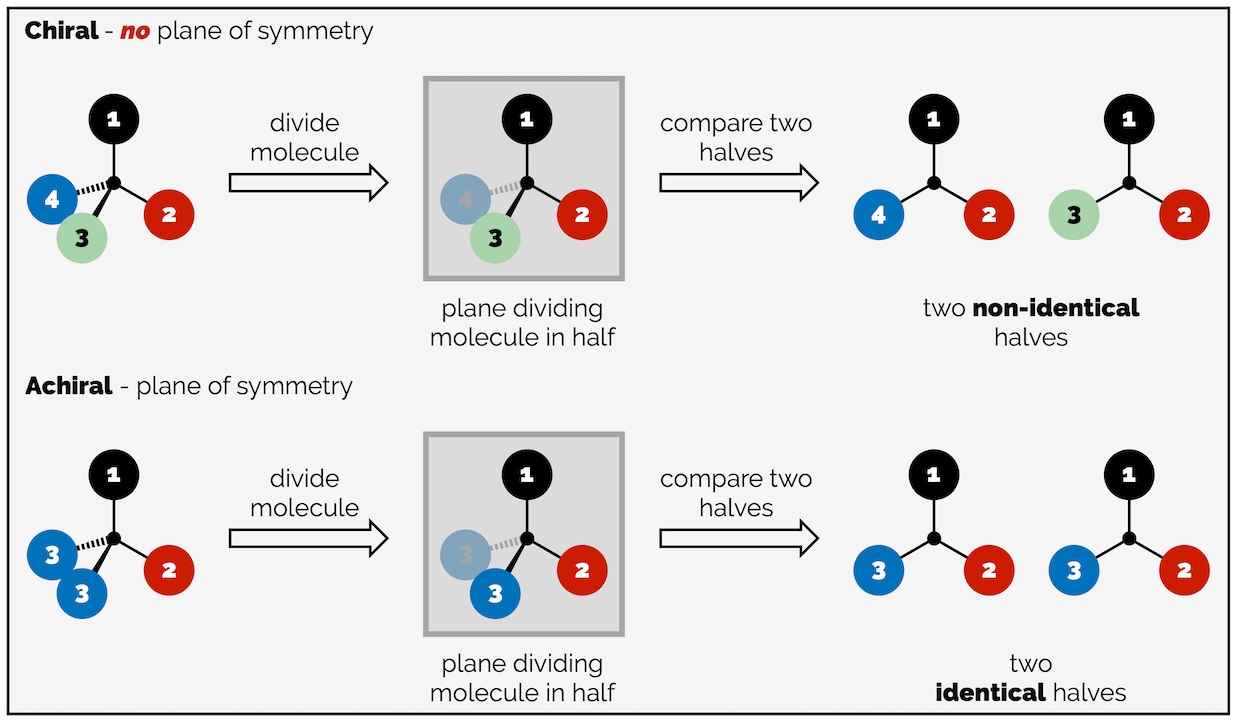
Two stereoisomers that are non-identical mirror images are called enantiomers. They are chiral objects. Any molecule that is the same as its mirror image is achiral. Chiral molecules lack a plane of symmetry. Achiral molecules possess a plane of symmetry. The simplest way to picture a plane of symmetry is to cut an object in half. If the sides are different there was no plane of symmetry. If they are identical (just reflections) then the object had a plane of symmetry. A molecule with a single stereocentre, like those shown above, will always be chiral. Those with two, or more, stereocentres can be achiral as they can contain a plane of symmetry (tartaric acid is a good example).
Chiral objects have no plane of symmetry. Achiral objects have a plane of symmetry. In this example, the plane is the same as the screen and cuts through atom 1, the central black dot and atom 2.
Enantiomers
A pair of chiral molecules, or enantiomers, are identical in almost every way. As they are mirror images all their properties are identical. They have the same functional groups and are subject to the same non-covalent interactions. Their physical properties, boiling points, melting points, solubilities and spectroscopic properties will be the same.
The only differences are in their interaction with plane polarized light, which they rotate the same amount but in opposite directions, and their interaction with other chiral molecules. Imagine your hands, chiral objects, grasping a straw, an achiral object. It doesn’t matter which hand interacts the result is the same, you pick up the straw. Now imagine your hands interacting with gloves, a second chiral object. Now there is a difference. Your left hand will only fit in the left glove. Two mirror images can interact differently with a chiral object. Why? The answer is diastereomers.
Diastereoisomers
It is possible to have stereoisomers that are not mirror images of each other. For example, cis and trans alkenes are stereoisomers but they are not mirror images. Stereoisomers that are not mirror images are called diastereoisomers (or diastereomers).
Diastereomers are different compounds
For a compound to be a diastereomer it must have two, or more, stereocentres as shown in the examples above. The configuration of at least one, but not all, stereocentres must be different. Compounds that are not mirror images are simply different molecules. There is no reason for their physical or chemical properties to be the same.
When two chiral molecules (or objects) interact you are effectively adding two (or more) stereocentres together, however temporarily. The presence of two stereocentres makes diastereomers possible. This is why the interaction of chiral objects can be different because diastereomers are different.
Relationships between stereoisomers
Stereochemistry can appear complicated due to the terminology used but if we look at one molecule and the various possibilities it should make a bit more sense. The molecule below is tartaric acid. You should be able to determine that it has two stereocentres. There are two carbon atoms that have four different substituents (don’t forget the hydrogen atom ignored in skeletal diagrams). This is a chiral molecule (or at least the stereoisomer I’ve drawn is …).
A diagram of tartaric acid showing the two stereocentres.
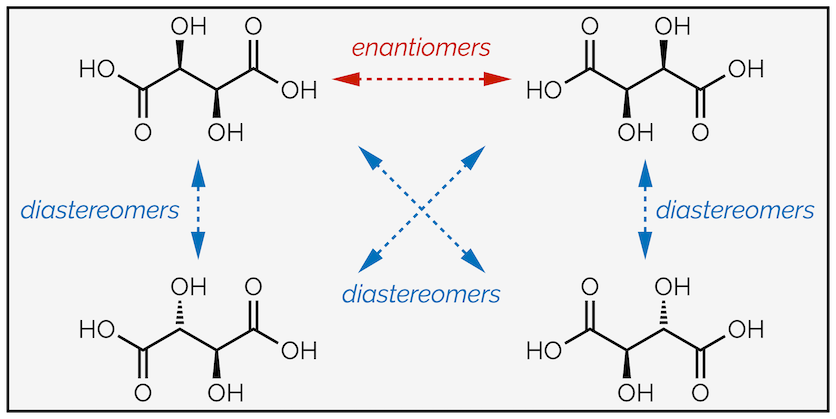
The stereoisomer above is a chiral molecule and it has a non-identical mirror image. It doesn’t matter how many times you twist or rotate the second image it will never overlap the first molecule. If you cannot see this, build a model of each compound (I recommend wine gums and toothpicks, they make the tastiest models). The mirror image is its enantiomer. The two enantiomers differ by their absolute stereochemistry or absolute configuration (the orientation of the atoms on the stereocentre) but they will have the same relative stereochemistry. This means the relationship between groups on the stereocentres is the same. For the stereoisomer below, both alcohols are on the same face, both upwards (or downwards, depending on how you draw the mirror image). A molecule can only ever have one enantiomer. Mirror images must come in pairs.
The enantiomers of tartaric acid
If you change the configuration of one stereocentre, and it doesn't matter which, you form a diastereomer. This is another stereoisomer but this time it is not a mirror image. It is a completely different compound. The absolute stereochemistry is different and the relative stereochemistry is also different. In one diastereomer both alcohols are on the same face of the molecule, in the other they are orientated in different directions. In other words, everything is different.
Stereoisomers of tartaric acid, showing both enantiomers and diastereomers
While a compound can only exist as two enantiomers, the original molecule and its mirror image, it can have multiple diastereomers depending on the number of stereocentres in the molecule. A molecule with a single stereocentre can be two stereoisomers (enantiomers). A molecule with two stereocentres can be four stereoisomers, two pairs of enantiomers. A molecule with three stereocentres can be eight stereoisomers or four pairs of enantiomers. In fact, if a molecule has n stereocentres it can have a maximum of 2n stereoisomers.
Various stereoisomers of simple sugars
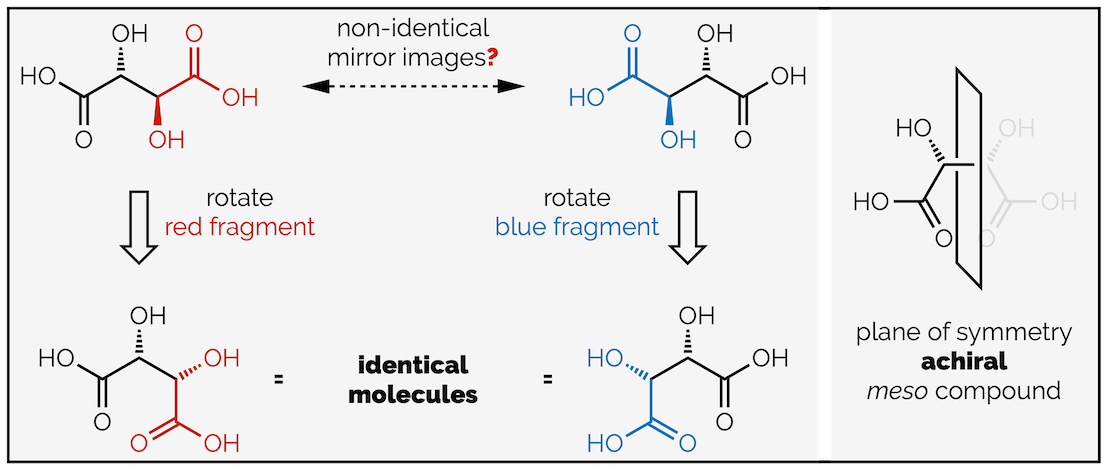
Note the word ‘maximum’ in the last sentence. Just because a molecule can have a certain number of stereoisomers doesn’t mean it necessarily will. Simplistically, chirality is the absence of a plane of symmetry. It is not a carbon with four different groups on it. If a molecule has a plane of symmetry it is achiral. Why is this important? Look more closely at the diastereomers of tartaric acid. Initially, you might think there are four stereoisomers; the two enantiomers at the top and two enantiomers at the bottom (of the diagram above), but there is a reason I didn’t add a red enantiomer label to the bottom molecule. If you rotate the central C–C bond, changing the conformation of the molecule not the configuration, you will see that they are actually identical mirror images or, in plain English, they are the same molecule.
Two depictions of tartaric acid are not diastereomers but the same, achiral compound (the same molecule drawn differently)
Such molecules, ones that contain multiple stereocentres but are still achiral are called meso compounds. You will rarely meet them at undergraduate but it is quite interesting …
Conclusion
The shape of a molecule is important, it can influence its physical properties and its reactivity.
There are many different terms used in the discussion of stereoisomers. Stereoisomers are molecules that have the same constitution (molecular formula) and the same connectivity (bonds between atoms). The molecules only differ by their overall shape and they cannot be interconverted without breaking a bond. A stereocentre is the atom in a molecule that leads to stereoisomerism. This means that if you exchange two of the groups connected to the stereocentre you will form a second stereoisomer. Diastereomers (diastereoisomers) are stereoisomers that are not mirror images. This means they have the same constitution, the same connectivity but are different shapes and are not mirror images. Diastereomers can be either chiral or achiral. Enantiomers are stereoisomers that are non-superposable mirror images or non-identical mirror images. They have the same constitution, the same connectivity and they are mirror images but they are still different molecules. Enantiomers must be chiral. A chiral molecule is a molecule without an internal plane of symmetry (non-superposable mirror images) while achiral molecules will have an internal plane of symmetry (superposable mirror images).
If you want some practice HERE is a worksheet with some problems.