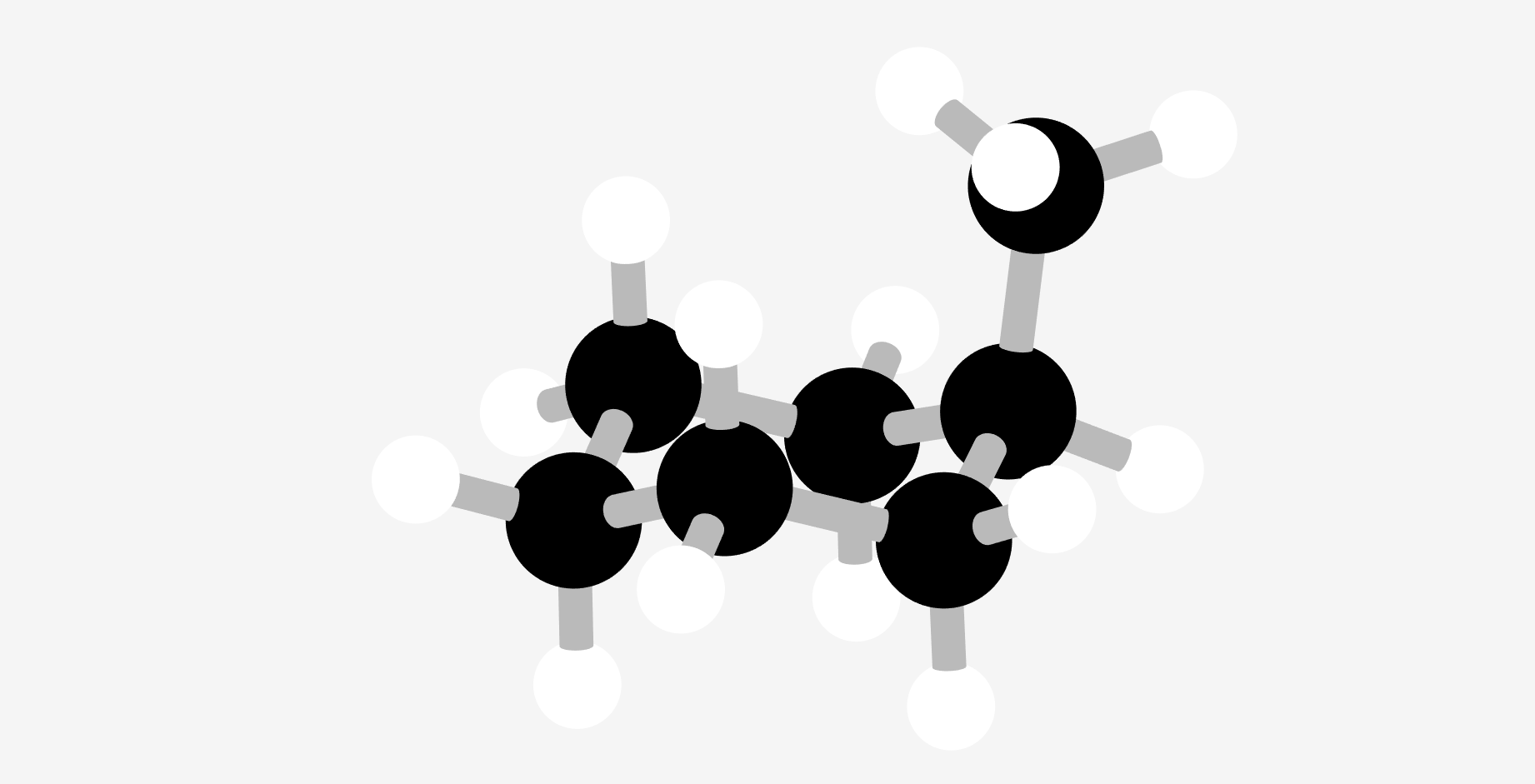
Stereochemical descriptors 3: Assigning a descriptor to a stereogenic plane
Having covered how to assign a stereochemical descriptor of both stereogenic centres and axes, it seemed to make sense to finish the organic stereochemical nomenclature with the descriptors for stereogenic planes (obviously, there is still plenty of nomenclature out there for people who like that kind of thing … metal complexes and some of the wonderful supramolecular constructs).
Annoyingly, there are multiple naming conventions for stereogenic planes with cyclophanes (and similar compounds) being treated differently from metallocenes. Worse, both of these categories can be further subdivided depending on use of stereocentres vs helices or just different naming systems being used simultaneously.
But here goes, hopefully it will be useful …
Stereochemical descriptors 2: Assignment of configuration for helical and axial chirality
Most chemists are reasonably happy assigning the stereochemical descriptors R or S to stereocentres, tetrahedral atoms with four different substituents. That confidence starts to drop off when we turn our attention to molecules with other ‘forms’ of chirality, be they planar, axial or helical chirality. This summary introduces how to determine the configuration of helices (using the descriptors P or M) and compounds with a stereogenic axis (using either the R/S terminology or treating them as a helix and using P/M).
An Introduction to the Conformation of Cyclohexane
Cyclohexane is the only strain-free cycloalkane. Understanding the chair conformation of cyclohexane, with its two steric environments depending on whether groups are axial and equatorial is an important skill. It can help you understand the shape adopted by biomolecules such as the carbohydrates. At more advanced levels, it forms the basis for simple transition models of many reactions and is used to justify the stereochemical outcome of reactions. This summary covers the basics, how to draw the chair conformation and how to place subsituents around the ring.
Conformations of simple acyclic alkanes
Molecules are not static. Single bonds can rotate causing the shape of a molecule to change. This is known as changing the conformation of a molecule. Some conformations are more favourable than others, these are sometimes called conformers. Other conformations are disfavoured. They are the barrier to rotation or a transition state between conformers. This summary looks at the conformations of simple alkanes.
Isomer Flowchart
The classic, what kind of isomer do I have here flowchart. There are a number of these online but it is useful. Surprisingly, hard to fit onto one page (nicely).
Stereochemical Descriptors: Naming Molecules
The name of a molecule must describe its structure including any elements of stereochemistry. This post introduces stereochemical descriptors for alkenes and simple stereocentres. Although I no longer teach this at undergraduate level, it should be useful for those that do. I’ve also thrown in some tips on manipulating drawings containing stereochemistry.
An Introduction to Stereochemistry
The shape of molecules is vital to understanding their chemistry and properties. The simple geometry of each atom is only half the picture, and you must also consider stereoisomers. These are compounds that have all the same atoms and bonds yet are different due to their shape. Students often find this a daunting topic. This introduction just lays out the basics. Sometime in the future, I’ll go into more detail (as I do teach a postgraduate course on this topic!)