An Introduction to the Conformation of Cyclohexane
Cyclohexane is the only strain-free cycloalkane. Understanding the chair conformation of cyclohexane, with its two steric environments depending on whether groups are axial and equatorial is an important skill. It can help you understand the shape adopted by biomolecules such as the carbohydrates. At more advanced levels, it forms the basis for simple transition models of many reactions and is used to justify the stereochemical outcome of reactions. This summary covers the basics, how to draw the chair conformation and how to place subsituents around the ring.
Conformations of simple acyclic alkanes
Molecules are not static. Single bonds can rotate causing the shape of a molecule to change. This is known as changing the conformation of a molecule. Some conformations are more favourable than others, these are sometimes called conformers. Other conformations are disfavoured. They are the barrier to rotation or a transition state between conformers. This summary looks at the conformations of simple alkanes.
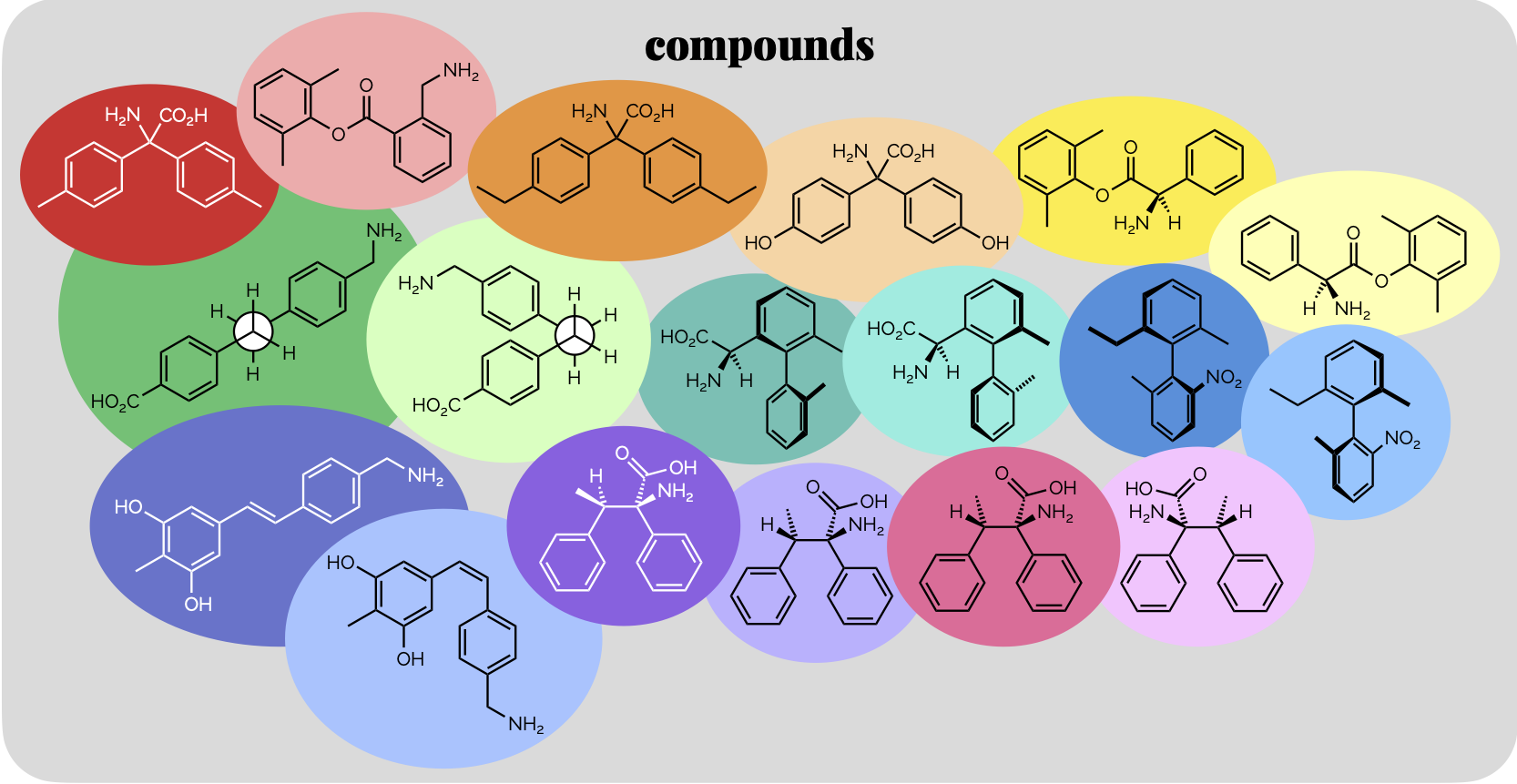
Isomer Flowchart
The classic, what kind of isomer do I have here flowchart. There are a number of these online but it is useful. Surprisingly, hard to fit onto one page (nicely).